
I can still remember a material science lecture back in college where our professor would discuss about metals and other engineering materials, then flash some images at different magnifications, since then I was fascinated at how things would look wonderful once viewed at a different perspective. Science and technology are indeed vessels to uncovering wonders.
As I transitioned from a student to a professional, my first industrial job was an electroplating engineer. Everything was new and surely fascinating, fascinating to a point that I’ve had several questions. Of course, I did some readings and I came across a study that triggered the “safety first” mentality in me. I’m really glad to share this information with you but before that, allow me to discuss briefly about electroplating.
Background
Electroplating was discovered and established centuries ago and have evolved since then. Primarily used to coat a thin layer of material (of desirable properties) onto another material (which lacks the desired property).| Electroplating is the application of a metal coating to a metallic or other conducting surface by an electrochemical process. The article to be plated (the spoon) is made the cathode (negative electrode) of an electrolysis cell through which a direct electric current is passed. The article is immersed in an aqueous solution (the bath) containing the required metal in an oxidized form, either as an aqueous cation or as a complex ion. The anode (silver) is usually a bar of the metal being plated. During electrolysis metal is deposited on to the work and metal from the bar dissolves. |
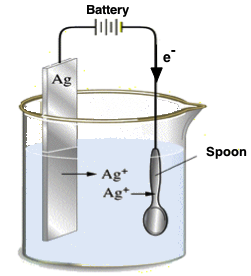 |
- Cyanide is an excellent complexing agent making it more tolerant to metal contamination.
- It can guarantees a pure white and bright deposit which are desired by customers.
- Inorganic plating, cyanide silver plating in particular are easier to control and to predict its behavior as compared to organic ones.
- Cyanide silver plating is undoubtedly very well studied making troubleshooting less difficult and puzzling. Answers to most of the plating engineer’s questions are readily available with due diligence and patience.
The Bad News
Today, a paradigm shift towards organic electroplating is gaining momentum as more engineers and scientist are formulating cyanide free bath which can compete with cyanide bath in terms of quality and ease of operation. This came about due to stringent environmental regulations and safety hazards that cyanide possess. Cyanide is on the Hazardous Substance List because as it is regulated by OSHA.
- Minute exposure can irritate the eyes, nose and throat.
- Severe exposure to cyanide can cause Cyanide poisoning with headache, weakness, confusion, nausea, pounding of the heart, coma and even death.
- Repeated lower exposure can cause nose bleeds and sores in the nose, and/or enlargement of the thyroid gland.
- Cyanide is toxic to many living organisms. Fish and aquatic invertebrates are sensitive to cyanide exposure. It can inhibit reproduction, causes delayed mortality, pathology, susceptibility to predation, disrupted respiration, osmoregulatory disturbances and altered growth.


Due to above mentioned risks, a large amount of investment is required to ensure a safe working environment and waste treatment prior to disposal.
Semiconductor industry is one that manufactures a wide range of electronic parts of various sizes where electroplating process is in no way excluded. Among the available metals, silver is widely used due to its outstanding electrical properties and solderability. Its downside is that it easily oxidizes or tarnishes, so it is usually over-plated with other corrosion resistant metals.
The Good News
Masaki et al., reported that several cyanide-free baths have been proposed, such as silver chloride-sodium system, silver nitrate-tartaric acid system and silver iodide system added with gelatin, however all these were deemed impractical for use. In their study entitled “Mirror-Bright Silver Plating from a Cyanide-Free Bath”, they found out that...a plated coat having performance comparable to that of cyanide silver plating bath can be obtained from silver methane sulfonate-potassium iodide bath to which N-(3-hydroxyl-1-butylidende)-p-sulfanilic acid (HBPSA) is added and has succeeded for industrialization; however, the plated coat obtained from this bath has poor brightness. A mirror-bright silver plating can be obtained by adding polyethyleneimine (PEI) as a brightener.
This breakthrough discovery is a wise, practical and sustainable alternative for the common cyanide bath. It addresses all the disadvantages of cyanide baths without compromising deposit quality. Preserving the excellent electrical conductivity and solderability of silver is more than enough to qualify as the best substitute. Addition of PEI only becomes necessary when deposit appearance is of value particularly in decorative coating.
I envisioned that more plating engineers will make use of this and publish their findings and claims. This is to close in the gap from cyanide plating where it has more conducted and published researches. A solid and intensive scientific foundation is all it lacks.
Kudos to Seishi Masaki, Hiroyuki Inoue and Hideo Honma!References
Text reference1. Seishi Masaki, H. I. (1998). Mirror-Bright Silver Plating from Cyanide-Free bath. Metal Finishing, 96(1), 16, 18-20.
2. Norman Greenwald, W. H. (2002). International Cyanide Management Code. International Cyanide Management Institute, United States. Retrieved from http://www.cyanidecode.org
3. Chicago Metal Finishers Institute. (2002). Non-Cyanide Silver as a Substitute For Cyanide Processes. Department of Natural Resource. Chicago, Illinois: The Illionois Waste Management and Research Center.
4. Electroplating. (n.d.). VIII Metals G Electroplating. Retrieved 2 9, 2018, from https://nzic.org.nz/ChemProcesses/metals/8G.pdf
Image reference1. Ag-100 silver nanoparticles 99.99% 20 nm, adjustable APS, dry powder or aqueous paste. (2018). Retrieved 2 9, 2018, from http://www.getnanomaterials.com/ag-100/
2. Chemistry - Electrolysis. (n.d.). Retrieved 2 9, 2018, from http://www.sciencequiz.net/lcchemistry/01_atomic/mcq/electrolysis/electrolysis1a.htm
3. OSHA Danger Sign: Cyanide Solution Contact with Acid creates Toxic Fumes. (2018). Retrieved 2 9, 2018, from https://www.mysafetysign.com/cyanide-solution-acid-creates-toxic-fumes-sign/sku-s-0077
4. Derla, A. (2013, 10 16). The Next, Worst Environmental Disaster in Europe. Retrieved 2 9, 2018, from https://alexandraderla.wordpress.com/2013/10/16/the-next-worst-environmental-disaster-in-europe/
This is a test comment, notify @kryzsec on discord if there are any errors please.
Being A SteemStem Member
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank you steemstem..
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congrats @jeebee0331! Now that this is what I call STEM!
Electroplating is such a useful breakthrough that has opened horizons to nanotechnology as it could be accurate down to the molecular level. But we have to overcome the environmental and health impacts first. I'm sure engineers like you are all up for that! :D
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I think that the amount of effort we should allot for environmental preservation must be twice that of technological advancement. For all we know, environment gave birth to science and technology,.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Couldn't agree more!! That's why we need more visionary engineers! 😊
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Good read. huhu akong upvote way value pa. haha.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
no worries.. happy nko nga gnhn mu sko post.. haha
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
GOOD
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank you
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations @jeebee0331! You received a personal award!
Click here to view your Board
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations @jeebee0331! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit