Greetings friends, today I want to continue showing how exciting is the area of industrial materials, being part of my training as a future engineer, in my previous publication I spoke a little about the ferrous and non-ferrous materials in that large group of metallic materials and the extraction metallurgy to obtain it, I will continue to talk about polymer materials focusing on the interesting thing that can become crystalline polymers, later we will see the wide world of ceramics and composite materials, I hope you like this material that I share.
We will begin by knowing that a polymer is a macromolecule that is caused by the union of one or more monomers. Polymers generally have a disordered structure, that is, amorphous, like consequence of the mechanism followed in the polymerization. An amorphous polymer is a polymer that due to the lack of regularity in its structure, can not form crystals, which require an order in the polymer chains.
Is the crystallinity in polymers more complex?
In the crystalline solids, the molecules are arranged in the three dimensions. This is what is called periodic ordering and it can have the crystalline solids constituted by small molecules. In the case of polymers, the chains are very long and easily become entangled and also, in the molten state they move in a very viscous medium, so they can not be expected in such a perfect order, but in any case, some polymers they exhibit partial ordering in regions called crystallites. A single macromolecule will not fit into one of those crystallites, so it bends over itself and can extend over several crystallites. While the crystallinity in metals and ceramics involves the arrangement of atoms and ions, in polymers it involves the ordering of molecules and, therefore, the complexity is greater. Polymeric crystallinity can be considered as the packing of molecular chains to produce an ordered atomic arrangement. The crystal structure is specified in terms of unit cells, which are ordinarily complex. Regions of two classes are distinguished: the crystalline ones, in which the chains bent several times in zigzag are aligned forming the groupings called crystallites; and other amorphous regions, in which the chains become entangled in complete disorder. The proportion or percentage of crystalline areas can be very high, as in polyethylene, in nylon and in cellulose. In those cases, it can be considered that the material contains a single phase, which is crystalline, although with many defects. In other polymers, such as PVC, the degree of crystallinity is much lower and it is more reasonable to consider it as two-phase systems, an ordered, crystalline, embedded in an amorphous matrix.
What is polymer crystallinity?
Polymeric crystallinity can be considered as the packing of molecular chains to produce an ordered atomic arrangement. The degree of crystallinity of the polymeric materials can vary from completely amorphous to almost entirely crystalline (up to about 95%). The metallic samples are almost always totally crystalline, while the ceramics are either totally crystalline or totally amorphous. The semicrystalline polymers have analogy with biphasic metallic alloys that is to say they present ordering regions and regions in which the polymer is amorphous. The density of a crystalline polymer is greater than that of an amorphous polymer of the same material and molecular weight, since the chains of the crystalline structure are more packed. The determination of the proportion of the solid which is crystalline (its crystallinity) is often of considerable practical importance, since an increasing crystallinity has the useful effect of improving properties such as strength and rigidity, resistance to dissolution and dimensional stability (thermal softening).
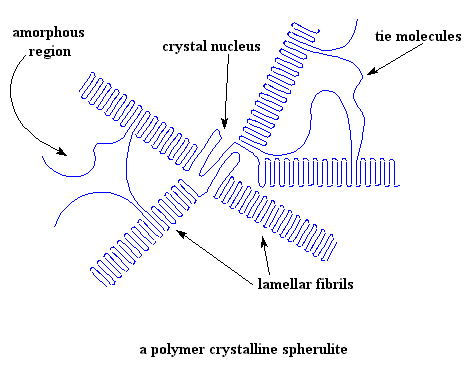
How to recognize when there is crystallinity?
The most direct test is provided by X-ray diffraction studies. The X-ray diagrams of the crystalline polymers simultaneously show sharp features associated with regions of three-dimensional order and more diffuse features characteristic of disordered molecular substances.
Configuration of polymers
The word configuration is used to describe those dispositions of atoms that can not be altered except by breaking and re-forming the primary chemical bonds. In 1932 Staudinger recognized that the monosubstituted olefin polymers should contain a series of asymmetric carbon atoms along the chain and there followed much speculation about the possibility of synthesizing such polymers in stereoregular form, such syntheses have been achieved by various ways . The regular structure of the resulting polymers, notably of the poly (α-olefins) was studied in 1955. The figure shows the polymer chain in its flat zigzag conformation, fully extended (all trans), the resulting configuration when all the constituent groups R of the vinyl polymer are left holm oak (or below) of the plane of the main chain is called isotactic. If the constituent groups are alternately above or below the plane the chain is called syndiotactic, while the sequence of random positions is said to be carried to the atactic configuration.
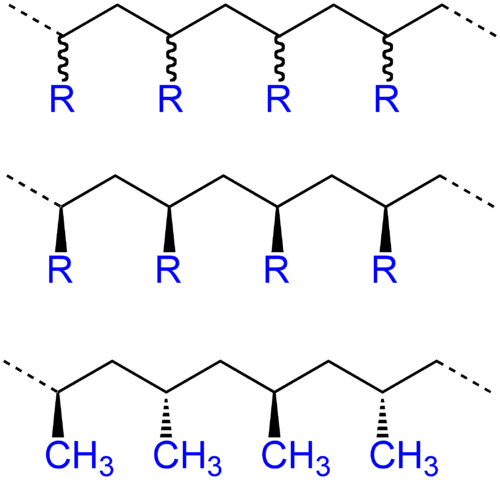
In a molecule in which only carbon atoms include the elements of the main chain provide a three-dimensional arrangement in the form of tetrahedra, this can be represented in several ways, one of them is the projection of Natta developed by Giulio Natta in which the zigzag carbons of the main chain are in the plane of the paper.
Tacticity can be understood only based on the study of stereochemistry is applicable only in polymers with chemistry centers in the main chain and because of this there are 3 possible arrangements: atactic (without order), isotactic (same order) and syndiotactic (alternating order) of the substituents in the chiral carbons. The characteristic of these links is that they are not superimposable in the third dimension, hence their representation on paper is a bit complicated. The sigma links (also known as simple links) of carbon can rotate with some freedom, but two enantiomers can not be overcome no matter how much this link turns, but it is necessary to break the links to relocate them and then superimpose them. A chemical model is very useful to study this type of links.
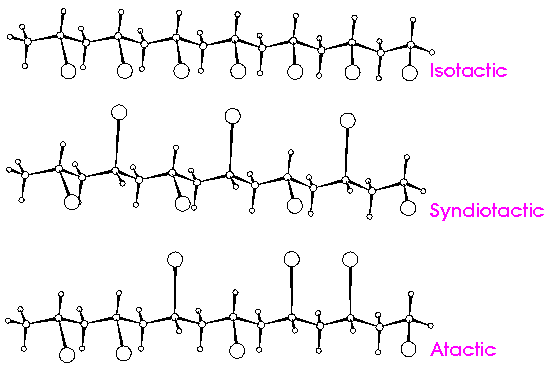
Isotactic polymers
A polymer with a stereoregular characteristic, in other words the functional group is located only on one side of the monomer, that is, on only one axis of the three-dimensional plane.
Syndiotactic polymers
The syndiotactic polymers also have a constant order in the substituents, however, they have inked alternately in the chiral carbon of the polymer backbone.
Atactic polymers
In atactic polymers, the substituents are distributed randomly along the chain of the macromolecule.
In the next part we will see examples of crystalline polymers such as esferelites, to which the crystallization of polymers is due and how this type of structure influences their properties. for more information consult the following references:
Fred W. Billmeyer (1975). Ciencia de los polímeros. Reverte.
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
https://www.scribd.com/document/48103160/Polymer-Crystallinity
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Great info... You remind me of college days when we were studying chemistry.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I'm glad you like it, university days are good that you remember what you learned.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Make sure to put copied content in block quotes and credit the source, or write original content if you want to avoid blacklisting
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks for the advice, I have to learn a bit of editing to avoid this type of inconvenience.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit