The Reckognition Of An Issue
My most recent series of posts required me to go through a huge amount of peer-reviewed papers. I have probably spent around 5-6 hours reading about 30 papers (granted, I did not read every tiny detail). For me, this series is about exposing myself to new approaches in science as I am trying to find an area that I want to specialize in. Currently I am working on a surface chemistry project trying to immobilize modified enzymes onto a self-assembled monolayer (SAM) of organic compounds that are fixed onto a gold surface. However, we are currently struggling with possibly irreversible contamination of our gold slides from airborne carbohydrates and residue from previous SAMs. This had me thinking, is gold a desirable carrier for proteins anyways?
Here my Steemit series inspired me to follow a different path. For one of my posts I read about nanocellulose and its amazing possibilities. It got me thinking. Maybe this may have an application to what I did and have been doing in laboratories for the last 3 years.
What Is The Issue With Gold As A Carrier?
Gold is a fascinating material with a shimmer that has captured mankind for probably thousands of years. Aside from that, it has very useful characteristics, such as very good conductivity and low reactivity under normal conditions. Its surface is ideal to attach polar groups to it through a process called chemisorption (according to my supervisor, we do not know if substances form an actual bond or if it is more of an attraction between different polarizations).
However, its ability to chemisorb polar groups may pose as a problem to fix enzymes onto its surface. Enzymes contain several polar groups, and they are usually concentrated on its outside. These polar groups are attracted to the gold surface and this attraction may cause the protein to denature (loss of shape, loss of function) if it gets too close to the gold surface.
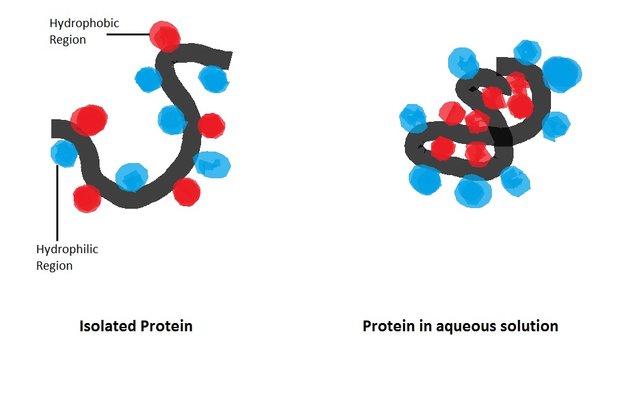
Cartoon illustrating the hydrophobic effect on protein structure. Hydrophilic regions are polar, hydrophobic non-polar. Gold can attract hydrophilic regions - Source
Therefore, you usually require a linker to immobilize a protein on a gold surface. In our lab we are using 11-amino-1-undecanethiol (MUAM) to place a rigid first layer onto the gold. This creates distance between gold and our enzyme. We then attached a second layer that consists of hydroxy polyethylenglycol (hydroxy PEG) and progargyl polyethylenglycol (P-PEG). Here is a simplified illustration.
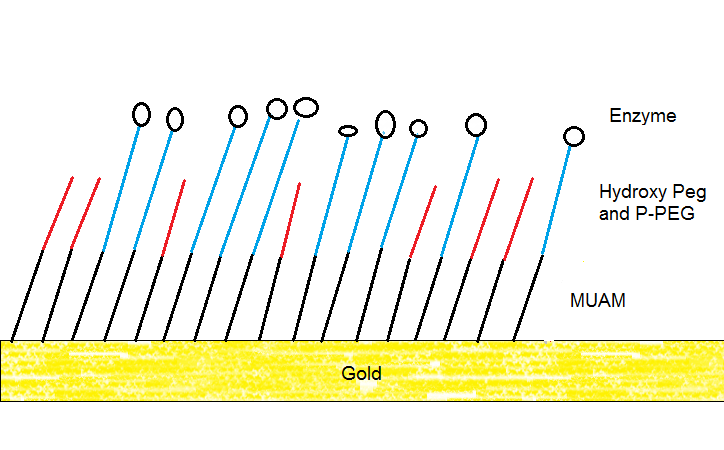
Cartoon illustrating the SAMs produced in my lab
There are several advantages of this system.
- It is easy to make and I can imagine it being scaled to an industrial level
- You require relatively few amounts of the compounds making your SAMs
- It takes only three days to create the SAM
- Quality control can be done using IR spectrometry
However, from my own experience, there are several disadvantages. The biggest one is that you need to make your gold carriers from scratch every time you because the gold substrates tend to bind airborn carbohydrates very easily See my Lab Chronicles - Day 6. Once they are contaminated, it seems like they cannot be cleaned. Therefore, filters based on this model would cause an increased need in precious metal, thus be expensive. Another issue is that they are not bio-degradable.
What Is My Idea, And Why Is It Better?
I want to use nanocellulose as a carrier for proteins. This has been done by several groups, however not in the same way as I am anticipating. Poly(acrylic acid)-modified poly(glycidylmethacrylate) nanocellulose has been used in the form of hydrogels to recover lysozyme from solutions(1). This approach can also be used for the recovery of amino acids, such as trypsin (2).

A superabsorbant hydrogel - Source
Nanocellulose can be modified in other ways by adding reactive functional groups onto its surface, enabling the addition of enzymes to its modified surface (3). The production of nanocellulose results in negative sulfate ester groups, that lend themselves for the adsorption of proteins and enzymes (4). It appears that enzymes can be directly adsorbed onto nanocellulose without the enzyme losing all its activity (the immobilized enzyme maintained roughly 67% of its activity)(5). I was actually very frustrated when I read the paper I cited in the last citation, because I wanted to do exactly what they have done, with two differences:
- I want to use polyethylene glycol as linkers and use click chemistry to covalently bond laccase to nanocellulose
- My goal is to create dry sheets of nanocellulose carrying enzyme (in this case laccase)
Laccase is able to oxidize various molecules with phenolic groups and thus has potential uses in biomedicine, biosensoric, industry and waste water treatment (5) (6). It is not picky and is able to use almost any estrogenic compound (estrogenic compounds all have a phenol functional group) that it gets in contact with (6).
I am currently in the process of formulating my research idea as part of my bachelor´s thesis. However, I am planning to submit it three months early and try to write and submit an actual research proposal to my university. Luckily, nanocellulose is currently getting very popular and industrial production of it is on the rise. I found a company that provides up to 1kg of microfibrils composed of nanocellulose as a free product sample (7), which means that I save 1000 dollar, making my proposal more likely to get accepted. Furthermore, the way they produce nanofibrils results in very large surface area and exposed hydroxy-groups, probably leading to easy modification of the surface.
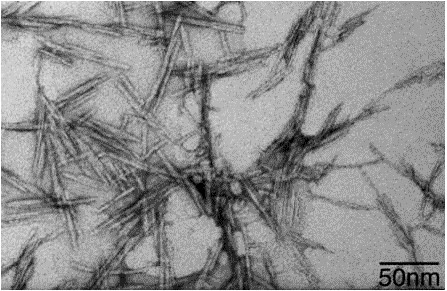
Nanocellulose fibers under electron microscope - Source
One of the advantages of my idea are that dry nanocellulose can be brought into any shape before the enzymes get attached to it. Thus sheets of modified nanocellulose can be made, enabling customers to bring the sheets into the shape they desire. Another advantage is that old tools build on the model I am working on would be a resource themselves. They could be reused by gassification (producing syngas) or by using them in bioreactors, producing energy making them a very sustainable product. I also believe that this is a very simple, 2-3 step process using chemicals that are readily available, once nanocellulose gets produced in greater quantities.
I know I am just a native beginner in science, but I feel like there might be some value to my idea and I am very excited to work on this project.
Shameless Self-Advertisement
Since this is my first serious research project I am designing from scratch, I would love comments. I am sure that some of you more experienced researchers find weak spots in my idea and I really want to hear about this so I can find a solution to that problem. Also, if you have a more advanced understanding of surface chemistry, chemisorption or materials science please help me out. I am trying to push this project through and possibly get a publication out and continue work on this in graduate school. Thank you very much for your help!
SteemIt Is Awesome!
I especially want to thank all the people from the SteemStem community, who have shown so much support. Another big thank you to the Curie curation guild who, like SteemStem, motivates me to provide quality content on a consistent basis. Steemit is such a great platform and it has already inspired and impacted my work!
If you liked this post please upvote and resteem. I am putting a lot of effort into posting daily to inform people about what is happening in the sciences.
As always,
Cheers @lesshorrible!


Your idea seems interesting to me. Good luck :)
I would just like to recommend you that you can also go for epoxidation of cellulose which will give the epoxy functional group to the cellulose and this epoxy modified cellulose can bind efficiently to cysteine group.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Hey @vinamra, thank you for your input. The professor that is working on modifying the enzyme I may be working with has the attachment all layed out so I know that if I can get the PEGs to attach, I will be able to attach the enzyme! Thank you though. Cheers!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Excellent post! @lesshorrible
I'm new to Steemit, it would be very helpful if you follow me and vote on my blog. @Fyahconde Check my content ... little by little I'll upload more and more.
Success and a Thousand Blessings for all.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit