Waste is polluting our planet. Bacteria can cause disease and spread death. Greenhouse gases can result in amplified global warming. Each by itself seems to result in nothing but doom. Let´s just mix them all together! The result, a possible solution to two of the biggest problems we may face in the future: waste, and global warming.
What Is This Supposed Miracle I Am Speaking Of?
Imagine a world where waste can be turned into something useful. A process that may be able to turn this into reality is pyrolysis.
Now, imagine a world were greenhouse gases can be used for good. We are developing methods to fulfill that promise also.
Another great imagination: bacteria that do not cause disease, but help us to produce useful good for society, on environmentally sustainable term. Look no further.
Biotechnology and chemistry are a great team. Sadly, as a former mentor of mine (who runs a biotech startup) said, there is not enough technology in biotech. However, research has resulted in some very promising new approaches. Today I want to talk about the production of syngas from waste, and the production of useful chemicals by anaerobic fermentation of carbon monoxide and hydrogen gas, facilitated by our arch enemy, the microbes.
How To Turn Waste Into Syngas?
Syngas is a mixture of carbon monoxide (CO), hydrogen (H2) and carbon dioxide (CO2). CO is toxic and CO2 is known to be a greenhouse gas.
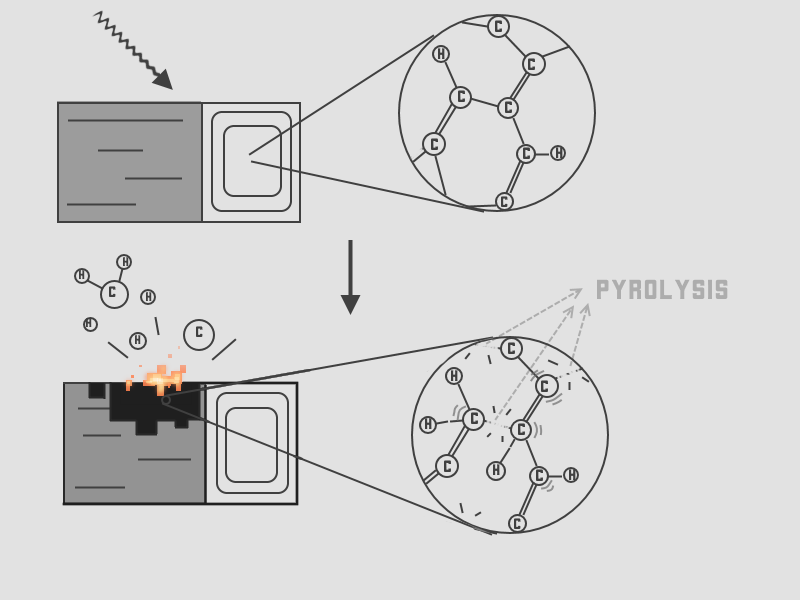
Solid waste, biomass (waste from agriculture, wood, fallen leaves, etc.) can be turned into syngas by a process called pyrolysis (1). Pyrolysis is simply the production of biochar (very roughly biocoal), by heating up waste, without burning it (2). Due to exposure to very high temperature, the molecules in the waste undergo decomposition (they break into smaller molecules), resulting in biochar and syngas. Atoms form bonds, this is a well-known fact. These bonds have specific energies. By applying heat (in the form of very high temperatures) you supply energy to a molecule, overcoming the energy of the bonds and thus giving the atoms incentives to go their separate ways. If you hit just the right temperature, you can cause complex molecules to split up in a lot of smaller molecules. This is done in pyrolysis.
Sadly, the conversion rates are not very high, but with new catalysts, it can be increased (1). In very crude term, we can turn waste into syngas by heating it up, while preventing it from burning.
But why should we bother about producing toxic greenhouse gases?
The Use of Syngas
Syngas has enormous value in the chemical industry. This paper demonstrates how to use syngas and a co-culture of two different bacteria strains to enhance the production of methane. This can be used as a fuel (methane is one of the main constituents of natural gas), or in the production of several other important molecules such as methanol.
Another way syngas can be used is in the production of ethanol, as shown in this article. Another study has demonstrated the same process, with an efficiency of 83% (3). Ethanol has long been used as a bio fuel and is also important in laboratory work. I have probably used 2-3 liters of ethanol within 6 days of laboratory work. It is very useful to dissolve certain materials and also important for cleaning laboratory equipment.
Syngas can also be used to form longer-chain hydrocarbons (4)
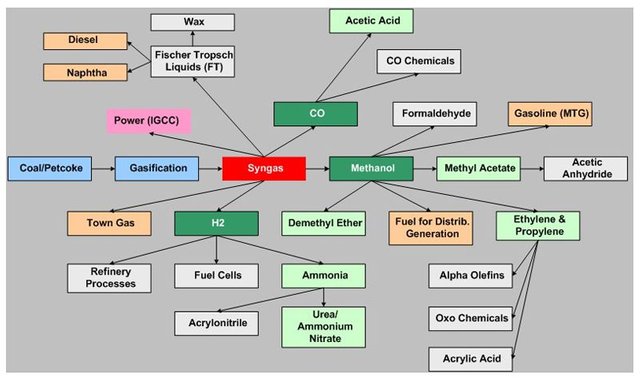
There are many other potential uses of syngas and its immediate products, as can be seen in this graphic.
Outlook
By developing new catalysts, we will be able to make the conversion of waste to syngas more sustainable, both financially and environmentally. Scientists are also working on developing new catalysts that are able to facilitate the conversion of syngas into other useful products.
The fantastic thing about this method is, that we are able to convert something useless, something harmful, into products that can benefit the world. Furthermore, by making this process more efficient and cost-effective, scientists are creating financial incentives for large scale industries to adopt this method.
I am a nature enthusiast, and I love being outside. So I really have an interest in maintaining nature, so we can keep impressions like this alive.
Shameless Self-Advertisement
Thank you very much for reading my post about this promising technology. If you have any questions please leave them in the comments. Do you think we may be able to take this technology to the next level?
I would also love to hear your input, if you have some more sophisticated understanding of this. I am a biochemist and chemist, not a biotechnologist or engineer.
I really appreciate quality feedback. If I did something wrong, please let me know and I will adopt it in my next post. If you think I did something very well - I always appreciate encouragements!
If you liked this post, leave an upvote. Also you may want to check out my series on instrumentation, my series Genius and Madness or my Lab Chronicles, in which I write about my research to develop filters that filter hormones out of water.
As always,
Cheers @lesshorrible!

.jpg)
This is really interesting!
I know lots of researchers had been borrowing the biology in bacteria to see how do they convert organic molecules to things like CO and CH4. After many years of evolution, there must be something we can take advantage of, but still, according to my knowledge, most of them are still not up to a economical level
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Yeah @mcw, I think this is tremendously interesting. I recently also saw a TED talk by two Canadians who found an economic way to destroy pthalates using bacteria! We are quickly picking up on using bacteria and fungi and I am really excited to see where biotech will take us. Cheers!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
You are talking about the one who found the bacteria in a bunch of plastic waste bottles right?
That was a really inspiring way to find out these digestive bacteria.
Yet, I am a bit doubtful about how effective it is, as doing these digestive process under a biphasic condition would be really slow?
Anyway, converting syngas to useful materials are really challenging, as most of the seminar I heard in the past few year all require precious metals (so people would say it is not environmental friendly, and expensive at the same time), and low TON is definitely another issue. So if cheap abutment metals like iron can do the job then i guess this would be perfect (bacteria use iron to do all sorts of oxidation too XD)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I am not sure if we are talking about the same. Here is the one I meant https://www.ted.com/talks/two_young_scientists_break_down_plastics_with_bacteria/transcript
I actually linked an article above where they state the use of ZnAl and copper-copperoxide catalysts to produce ethanol in a one step synthesis. That sounds very intriguing. Here is the article http://pubs.acs.org.ezproxy.samford.edu/doi/10.1021/jp502828c
Cheers!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Oh! I guess the one I saw previously is this
I just quickly go through the acs paper and it seems more like a theoretical one to me. They seems not show the catalyst loading and the turn over number (seems not in the SI as well). I am not an expert in catalysis so I cant comment too much, but I still think this is really a “hot field” in the coming decades ~
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
No that one specific paper is only showing that it is possible. I linked another one in the post where they reported 83% efficiency. Though, I have to admit I did not read it in its entirety (after all, I only got so much time during the day). Yeah it will be interesting. My interest right now tends more in the direction of instrumentation, and nanotech. Dude, I really appreciate your interest and contribution! I sadly can only give one upvote...
Cheers!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
This is the one i came across sometime ago but is quite impressive. Although using ruthenium catalyst, it is capable to do CO2 reduction (personally I think this is a pretty nice yet difficult to do CO2 reduction to give useful materials) with high TON. Just put the link here if you are interested
http://onlinelibrary.wiley.com/doi/10.1002/anie.201500939/abstract
Enjoy :D
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
That is also very promising. I am sure that these efforts will yield some very interesting procedures and applications. Cheers!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
good post friend and you vote greetings, friend if you wish you can see my last post and if this is of your interest you can retest me thanks greetings.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
This is promising. I knew scientists are not just waiting global warming to past by itself :) Hope we can see more developments about his technology in the future. Thanks for the sharing by the way :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
You are welcome @cihaddogan! Apart from preventing global warming, I believe that re-using materials such as waste is very efficient. Even if in this context we find methods that are not too useful for recycling waste, we may be able to use that knowledge in another area. That is the good thing about science and new technology, any new discovery can be useful. Global warming has one upsite, it has increased the attention that research receives and thus more funding, and more results. Cheers!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Really good Post! I Blog about waste and Recycling! Maybe we can work together :) Upvote and Follow!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I really appreciate your comment at @leranion! I am not necessarely too keen on posting about recycling. Don´t get me wrong, I am all for recycling and I do it myself, but I am focusing on posting about my own research, as well as new research that I find interesting. So, I am sure there will be more posts about green chemistry and I would love your comments! I do like your blog though, so I am following you back. Cheers!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
good writing and content! my company is working on similar projects, I posted about recycling gold with bacteria, watch my blog :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
That is awesome! I read that you live in Heidelberg! I am also from Germany haha, was für ein Zufall. Right now I am working on my bachelor´s degree but I am hoping to get into a joint masters in surface, electro, radiation and photochemistry as part of the Erasmus plus program. So, I am hoping to start my own biotech/chem company in the future. I will definitely check out your blog! Glad to meet fellow scientist. Cheers!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Way to go buddy thanks for continuing to blow our minds. You're letting everyone know what you're learning in college which is like giving us a free college education
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Haha dude, I wish I learned this in college! This is something I researched because I was intrigued by a TED talk. I am hoping you find your project soon. Cheers!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
good!.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
happy today for you!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit