To learn about the LION experiment please start with this video and look for any other related videos on the MFMP YouTube channel.
Untreated virgin 3M Green Diapad 'Cookies'
The industrial diamond in Nickel disks were taken from a green 3M Diapad abrasive pad which is available internationally for the purpose of rounding the edges of cut glass sheet amongst other uses. MFMP volunteer Alan Goldwater in California shows how to easily remove them from the pad.
In the following video, you can see the so-called 'cookies' up close. Video was taken with a NURUGO Micro lens attachment for smart phone based microscopy on a Samsung S7
Analysis
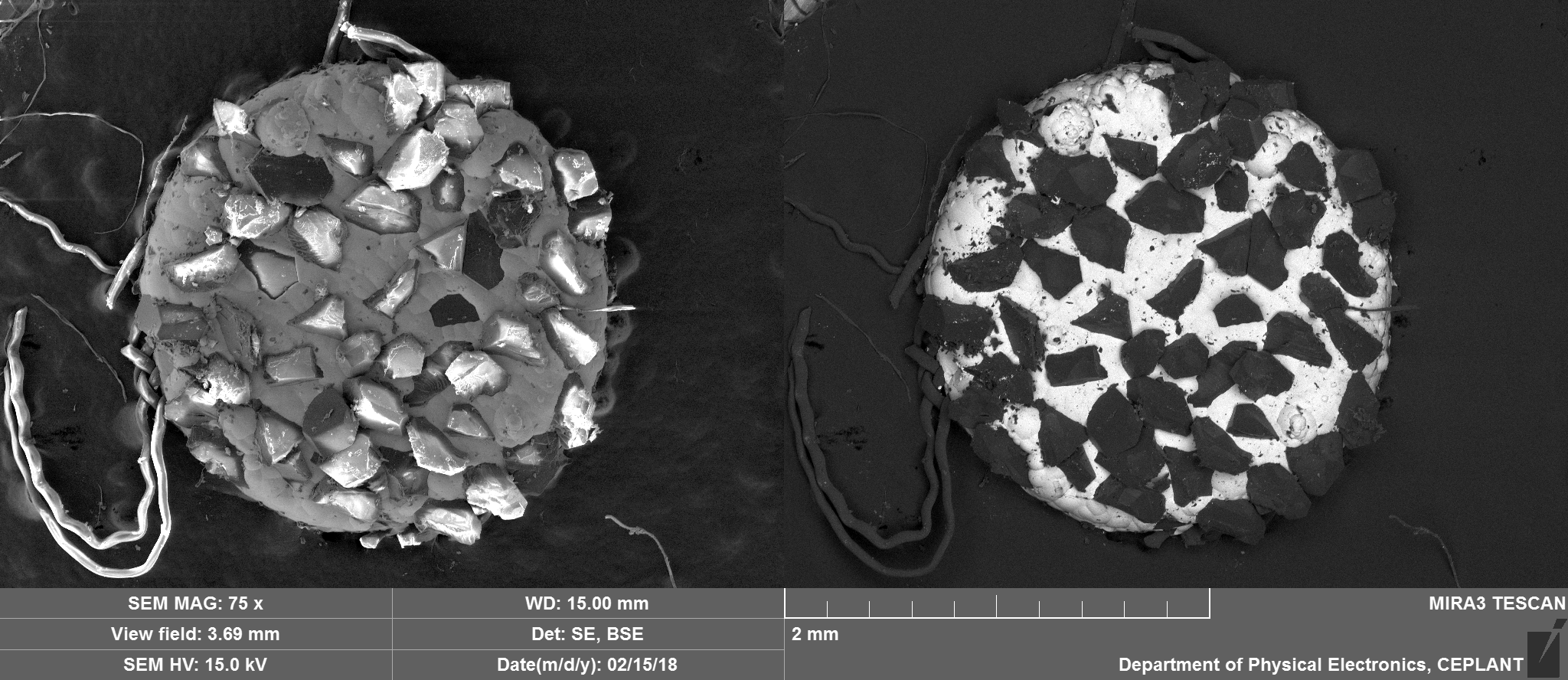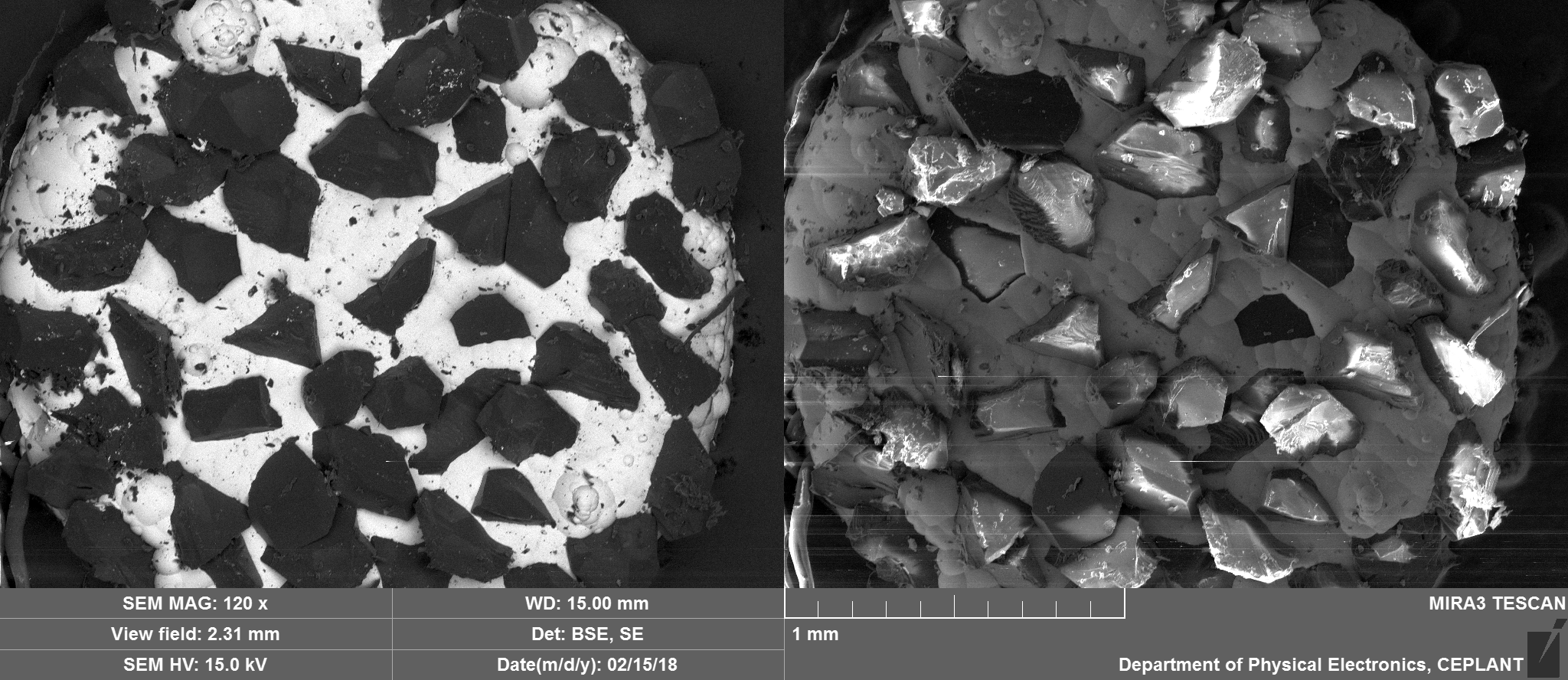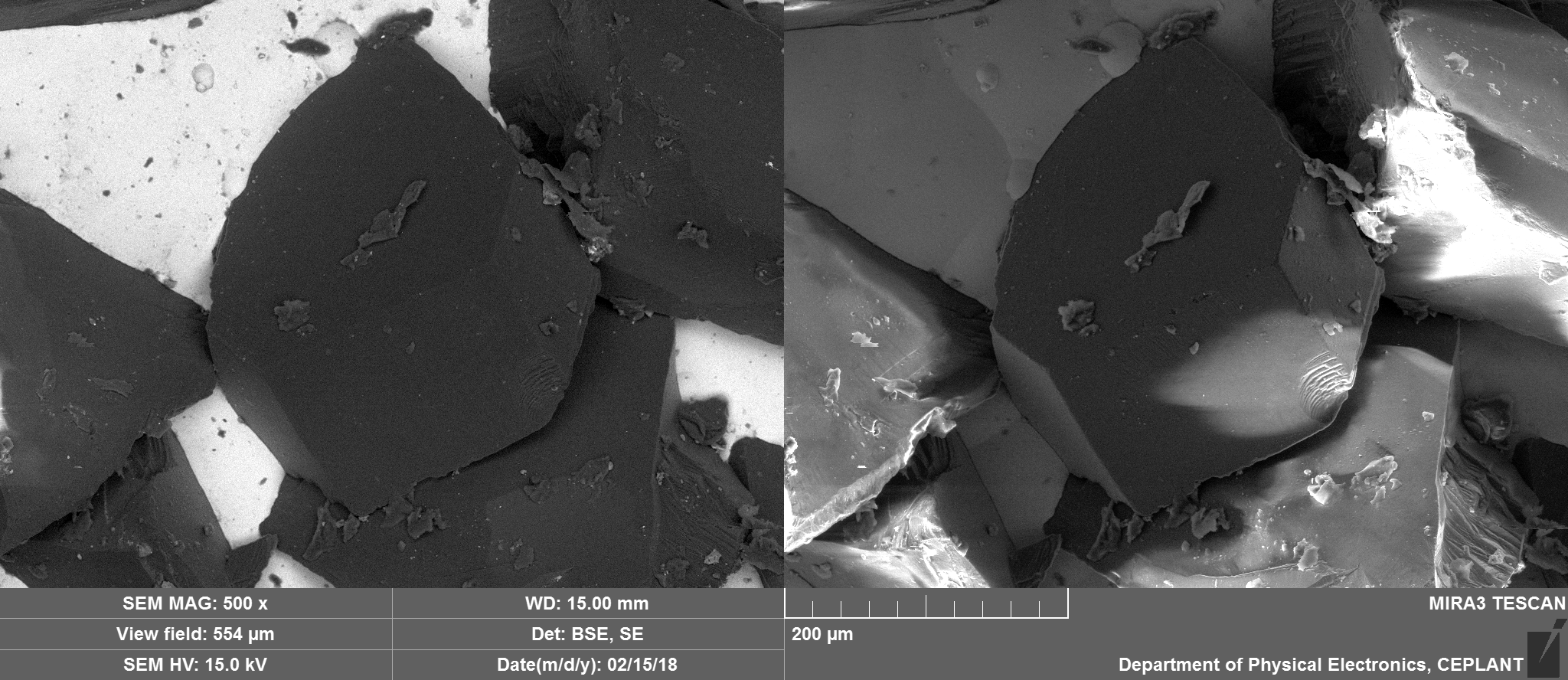
They can be seen in the image below circled in red, prepared to go into the scanning electron microscope. They are approximately 2.5mm in diameter with variation often mostly due to some diamonds jutting out from the nickel that holds them in place.
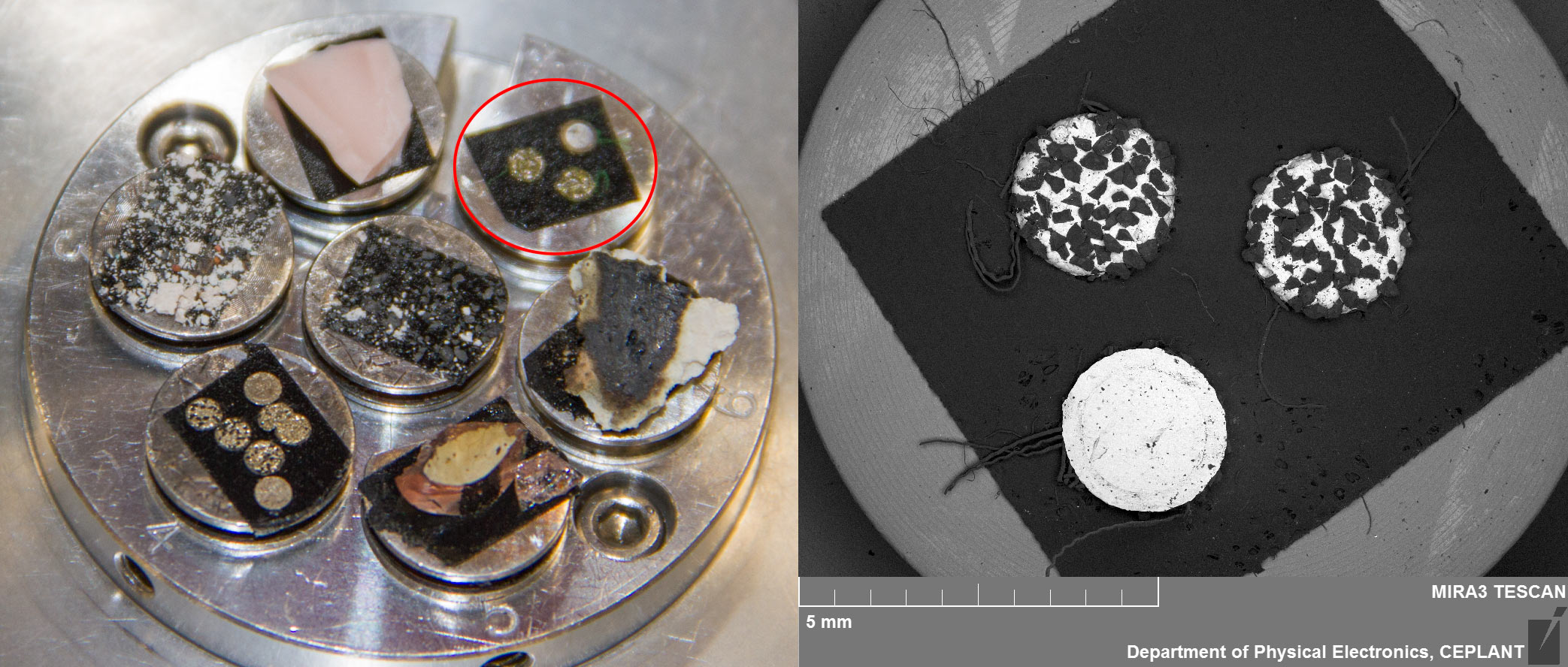
Top Side Overview
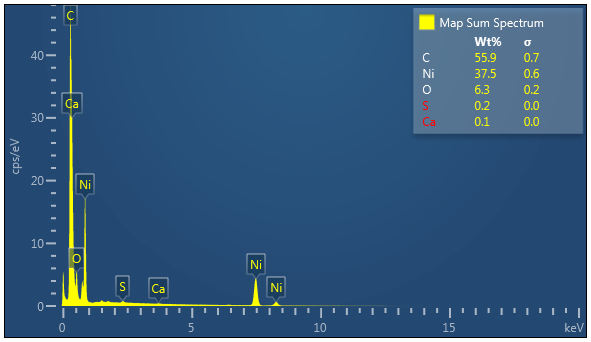
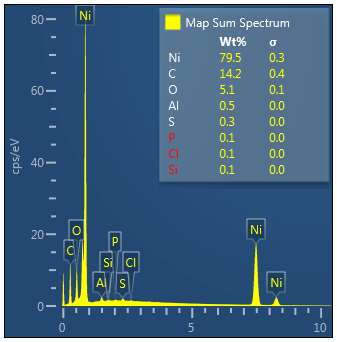
Nickel, Carbon, Oxygen and potentially traces of Sulphur which appear to be located on the Nickel
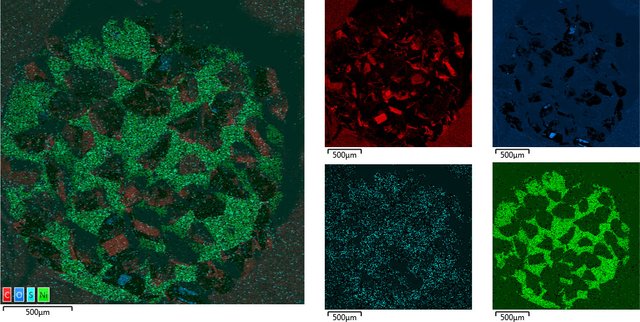
Zoom in on Diamond tip

Essentially just Carbon with potentially Nickel and traces of oxygen.
Backside

'Cookies' post LION 2 experiment
Diamond surface changes
MFMP volunteer Alan Goldwater did some investigation about the effect of Oxygen on Diamonds at 800ºC, he found the following paper
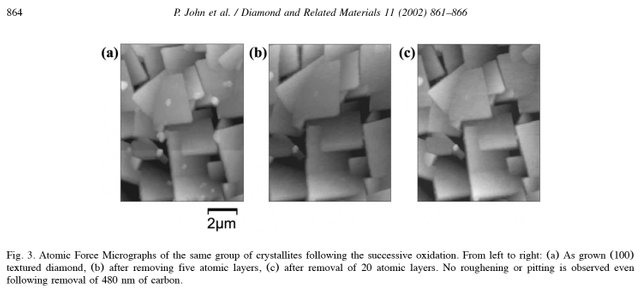
Oxidation of Diamond: John, P.; Polwart, N.; Troupe, C. E.; Wilson, J. I. B. (2002). "The oxidation of (100) textured diamond". Diamond and Related Materials. 11 (3–6): 861.
It contains the following image showing how diamonds are degraded
Alan Goldwater summarised his findings based on this as follows:
The above paper seems to find that the oxidation happens one atomic layer at a time, at a rate proportional to the O2 partial pressure.. The O2 etching is such that the surface morphology (ie. facets and points) is exactly preserved. The experimental measurements found a rate (at 1 bar 800°C) of ~1 nm/sec. Thus for a hypothetical 2 day run finishing at 800°C for 4 hours, the erosion time might be 15k sec, or 15 microns of oxidation. The 3M product spec is 250 um (250M) grit size, so the erosion from oxidation would be around 6%.
What actually happened to the diamonds in LION 2?
From colour optical microscopy it was obvious that the vast majority of the diamonds were not there any more and those that were, were now black and degraded. Also, there was some degradation to the Nickel substrate and a selection of 'spheres', mostly black.
Frontside
Backside
Analysis
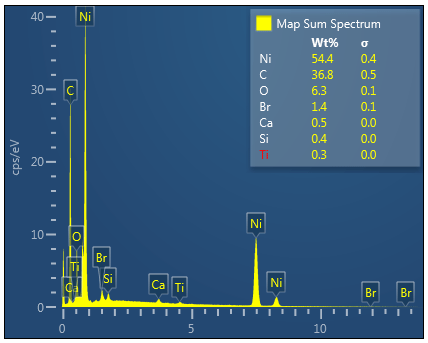
Bromine (Lα 1.480) as shown in the table below was manually determined to be an incorrectly automatically determined element and was swapped for Aluminium (Kα 1.486) See EDS energy table here

LENR Reactions or contamination from fuel tube?
| George Ohsawa reactions? | Elements detected in fuel tube? |
|---|---|
| C + O > Si | Fe |
| 2 * C + O > Ca | C |
| Deuterium reactions? | Al |
| (2 * C) + D > Al | Mg |
| 2 * (C + D) > Si | Ca |
| (3 * C) + (2 * D) > Ca | Si |
| (2 * 13C) + 12C + 4D > 46Ti | F |
| (3 * 13C)+ 4D > 47Ti | O |
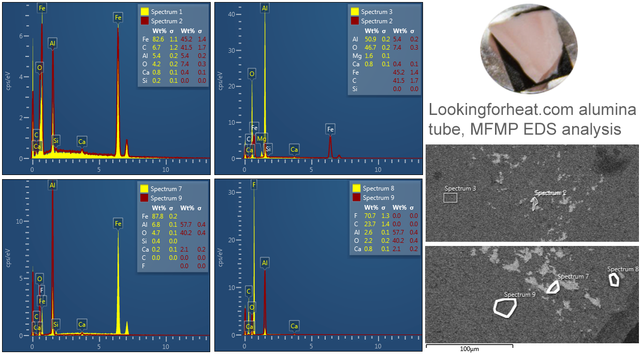
Oxides of metals found have very high melting and boiling points, so is it likely they will have migrated?
| Element/compound | Melting point ºC | Boiling point ºC |
|---|---|---|
| Magnesium | 650 | 1,091 |
| Magnesium Oxide | 2,852 | 3,600 |
| Aluminium | 660.3 | 2,470 |
| Aluminium Oxide | 2,072 | 2,977 |
| Calcium | 842 | 1,484 |
| Calcium Oxide | 2,572 | 2,850 |
| Silicon | 1,414 | 3,265 |
| Silicon Dioxide | 1,710 | 2,230 |
| Iron | 1,538 | 2,862 |
| Iron (III) Oxide | 1,565 | 2,623 |
| Titanium* | 1,668 | 3,287 |
| Titanium Dioxide* | 1,843 | 2,972 |
*Not detected in reactor core starting materials
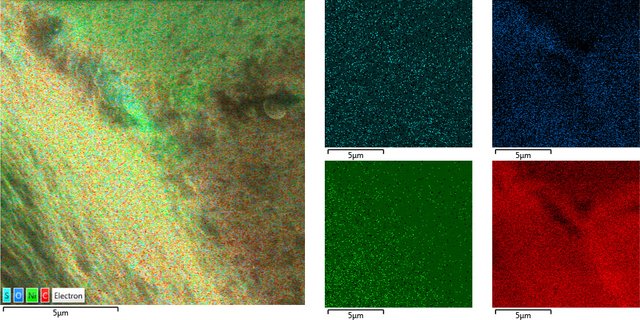
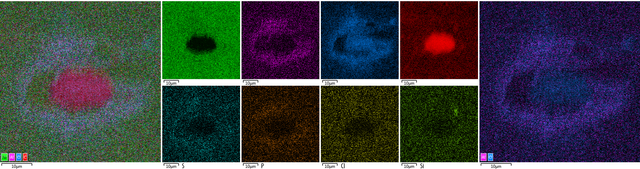
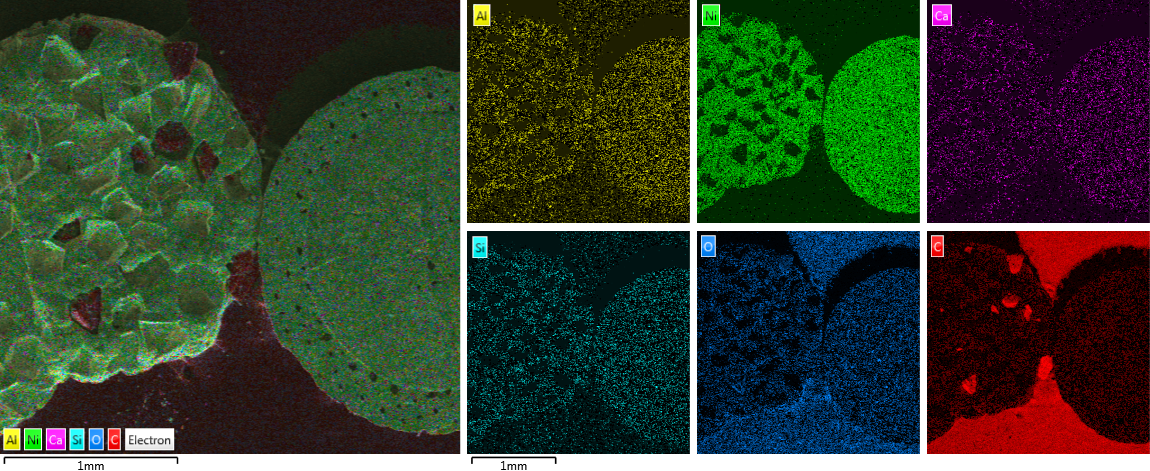
Placement of detected elements on diamond surface
Diamond 'Erosion' patterns
Diamond element placement
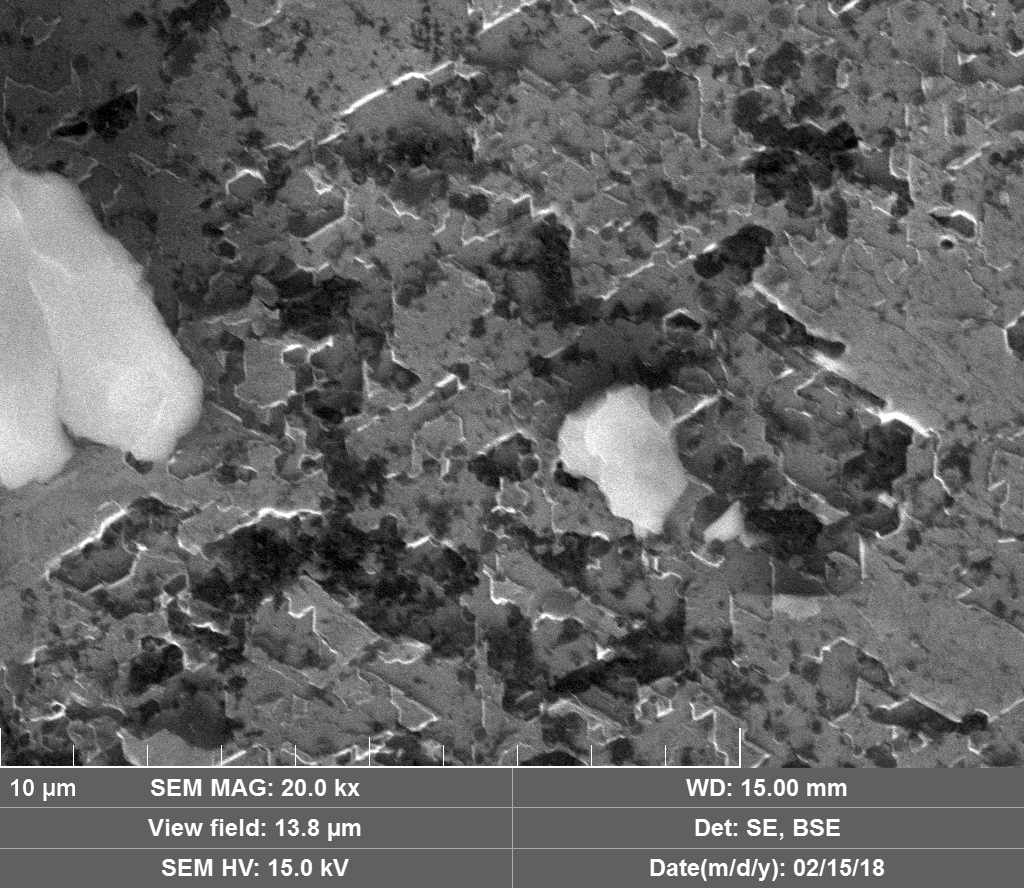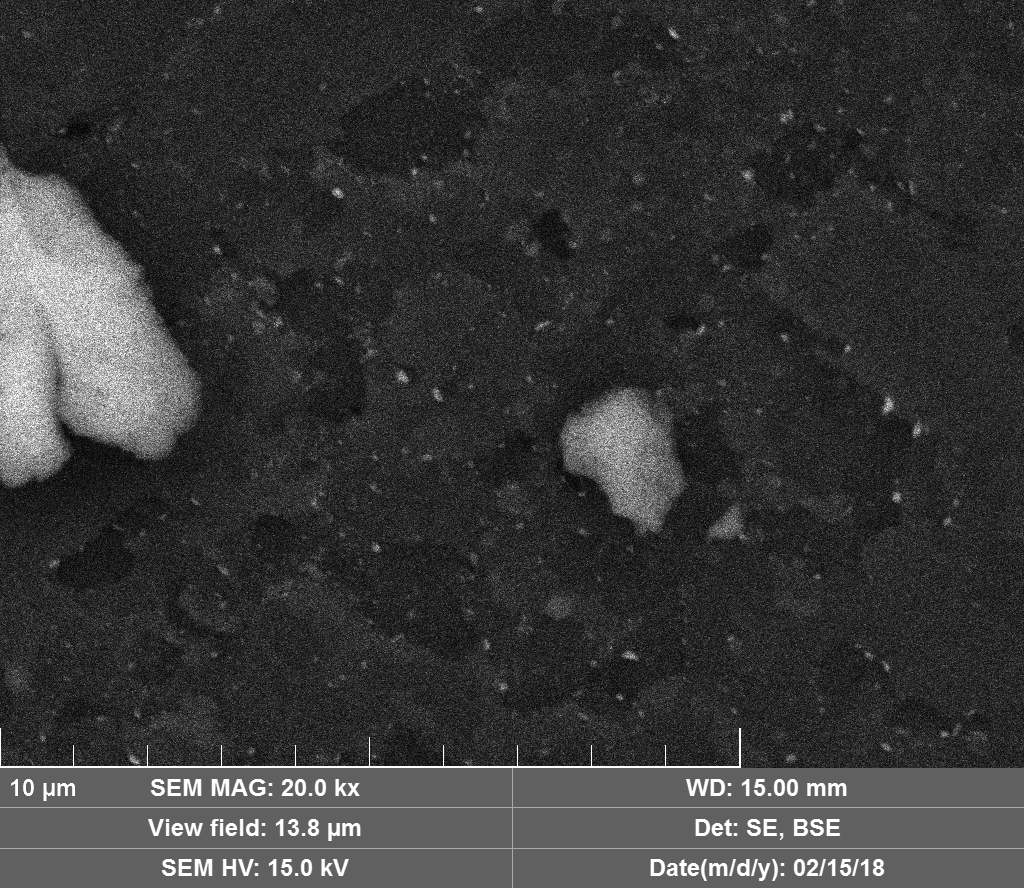
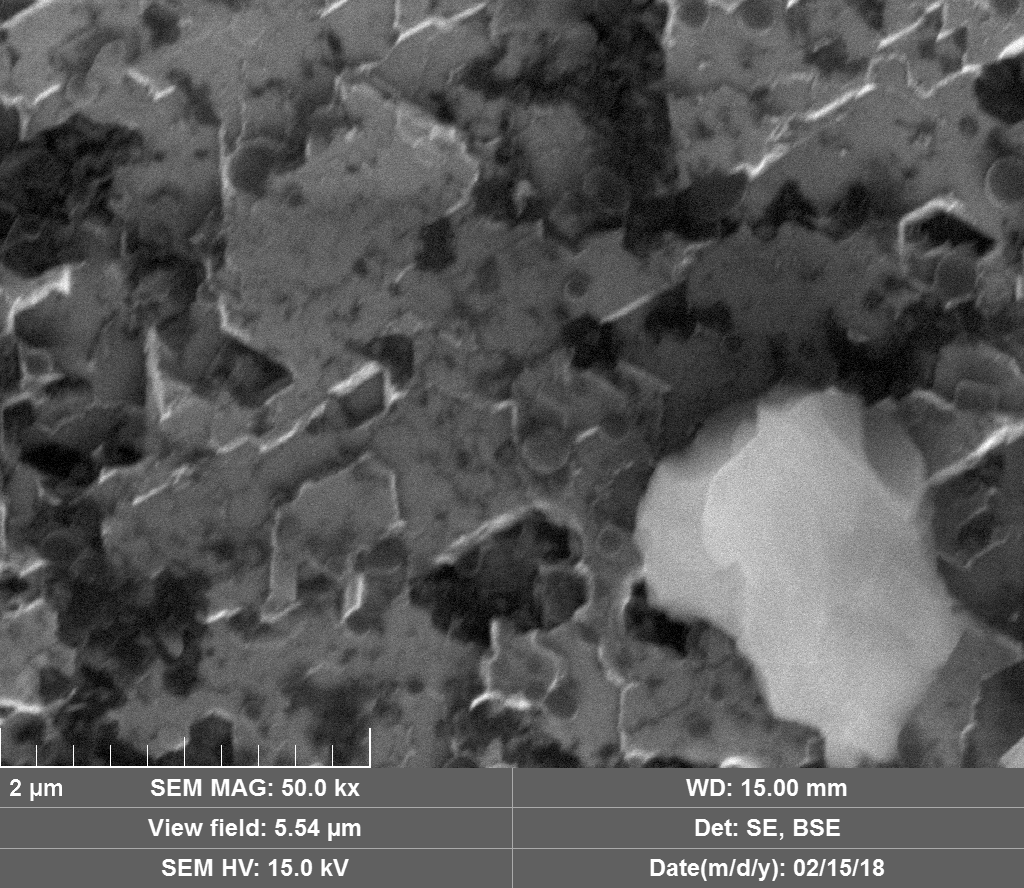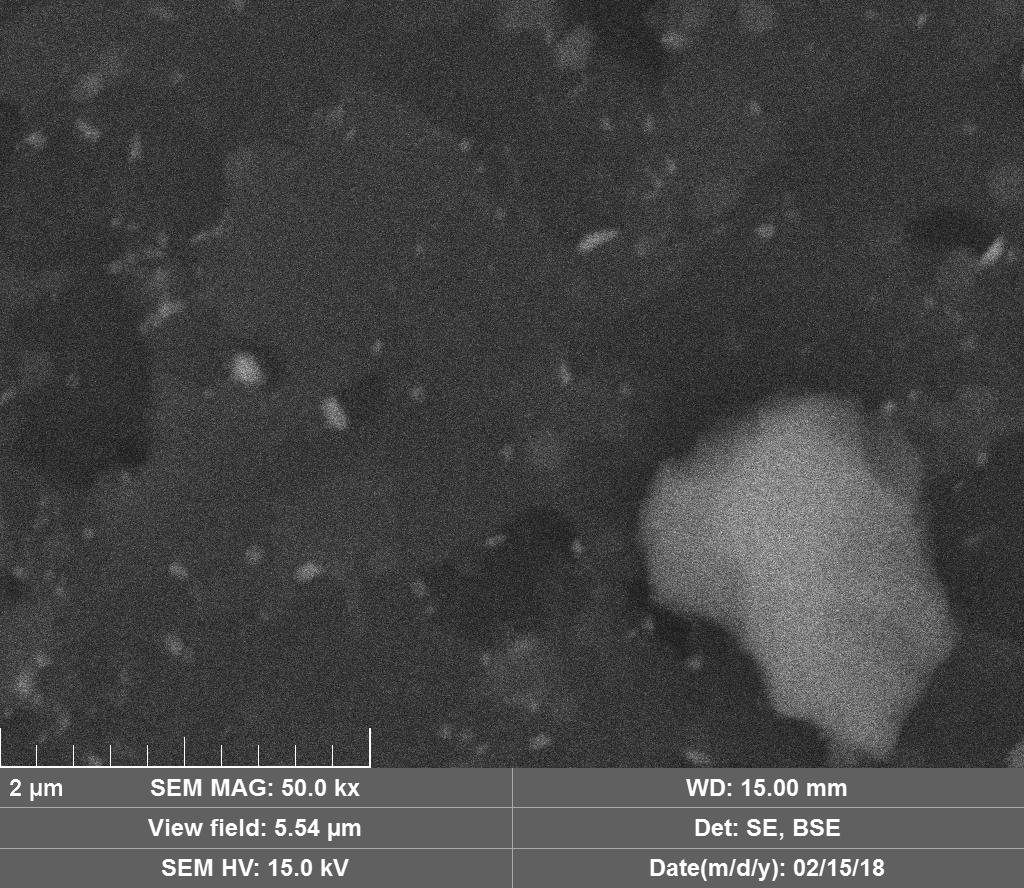

Discussion

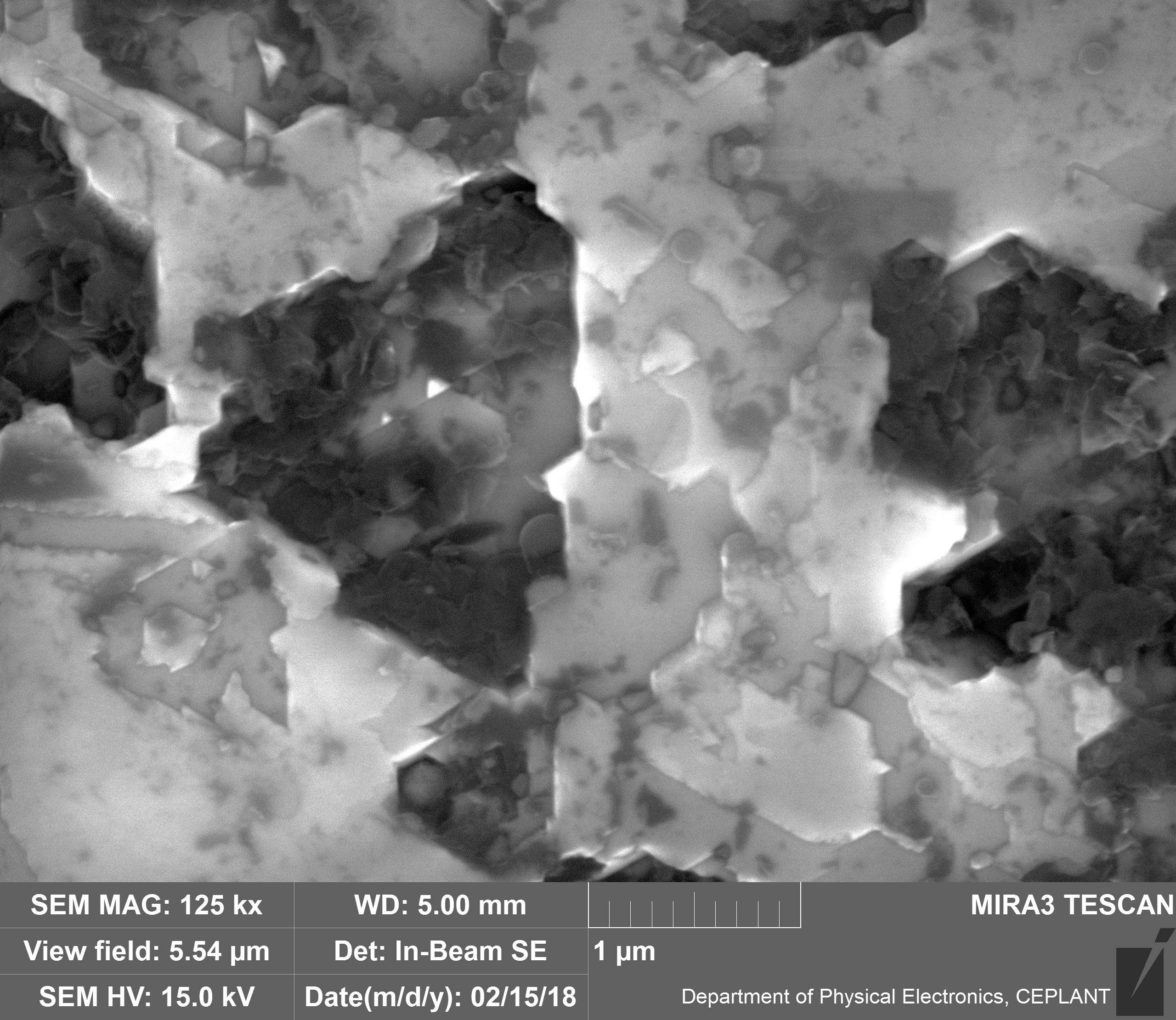
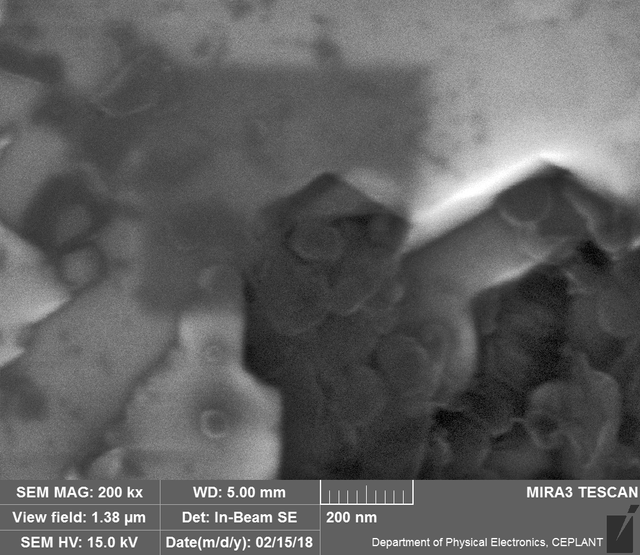
Upon visual and high resolution photographic inspection, the inside of the LION 2 core tube, apart from 'the pile', looked fairly clean and untouched, suggesting that it had not been significantly damaged by the LION reaction. On the other hand, the diamond in the heavy water soaked baked 3M Diapad cookies was either not there or very significantly eroded in what looks like an active component acting in specific ways rather than the normal global removal of Carbon as discussed by John, P. et. al. in the "oxidation of diamond" study cited above.
In the diamond there is often discrete 'spheres' sitting in cavities or at the end of 'tracks', also, there is wetted smooth blobs with similar relationships to erosion patterns. Normally it appeared that these were different elements based on the backscatter image. Sometimes 'spheres' appear to have a similar backscatter signature to the diamond suggesting they may largely be composed of Carbon, if this is the case, this raises the question of how the carbon, which has a melting point of 3550ºC is forming discrete 'spherical' inclusions.
The erosion pattern looks like it follows 'seams' or plane boundaries in the diamond - often changing directions. There are cases however where the 'mining' cuts across the planes or through the bulk. In other cases, it appears that there is a 'sphere' in the centre of a cavity that is hexagonal in shape as if the diamond carbon has been pulled to the centre.
It has been noted that the main elements identified are present in very small localised quantities, likely as oxides in the reactor tube. The melting point however of the element oxides detected in the Alumina reactor tube are well above the temperature that the reactor is able to be heated to via electric resistive heating, that is nothing to say for the boiling points. Moreover, there were areas of near pure Iron found in the reactor tube however there is no iron found on the diamond when there is silicon. Likewise, there was magnesium (presumably as an oxide) found in the raw reactor tube but not on the diamonds.
If, in some way the elements are coming from the reactor tube, how is it that they coalesce into the cavities? If there is no process that creates the elements found in these cavities, how then do the cavities form to host this material? Use of a higher purity reactor tube or one composed of Boron Nitride would eliminate this concern.
Conclusion
The heavy water soaked baked diamonds in the LION 2 reactor appear to have undergone atypical erosion when compared to temperature / pressure diamond oxidation studies. In many cases, the diamonds have disappeared only to be replaced by 'spheres' or blobs of heavier elements.
From the physical and distribution evidence, it appears that in the core of the LION 2 reactor the active structures were 'mining' the deuterated industrial diamonds and converting it into Calcium, Aluminium, Silicon and potentially Titanium. If this is verified by further study and replication with correct controls in place, it is an extraordinary finding.
For those interested in an open working New Fire reactor becoming available, please consider a PayPal donation here or via our MFMP Donation page where your name will be recorded in the record of donors.
The project looses 4.5% on Paypal donations, so for those that can do a direct bank transfer, donations of any size can be made direct to Quantum Heat C.I.C.s (the organisation that runs the MFMP) bank account here, thus saving charges:
BANK: HSBC
Branch Address: 153 North Street Brighton, East Sussex, BN1 1SW
Account Name: Quantum Heat C.I.C
Sort Code: 40-14-03
Acc No.: 12631571
IBAN: GB48MIDL40140312631571
SWIFTBIC: MIDLGB22Or bitcoin: 1JesTRUXU8jHb1haa1MoF2NvrU8gt5msCr
Alternatively there is a US 501.c3 that also supports the goals of the MFMP, you can request information on how to contribute by that route by writing an email to [email protected]
This is an interesting paper, shared by contributor 'Ahlfors' on LENR-FORUM and highlighted by 'Stephen' on ECW - especially considering the use of Nickel and Diamond.
Discussion
Firstly, it is based on the immersion of the Diamonds in 12-52μm nickel powder, Nickel of course is not present in the powder form in LION. Having the nickel in this scale will allow sintering processes at 600ºC and lower perhaps - so the Nickel becomes mobile and catalytically active.
Secondly, whilst there are nano particles seen in the cavities, there is no suggestion that they are composed of anything other than Nickel, Carbon and oxygen.
We did not detect Boron in our diamonds, perhaps limitation of the analysis - however, the study does state that its diamonds contain Boron.
Perhaps the Deuterium Oxide changes the catalytic dynamics?
Further, there does not appear to be the abundance of 'spheres' forming on the surface of the diamond.
It could be that in LION 2 - the Nickel catalytically splits D2O releasing some O that forms some CO with the diamonds C and this in turn chemically transports some of the Ni via the Mond process that then deposits itself on the diamonds and the nano nickel particulates so made subsequently lead to a similar process to that which is cited in the above linked paper - however, this does not account for the other elements and 'spheres' found on the LION 2 'cookies'.
In LION 2, there also does not appear to be any 'molten nickel' type structures on the diamond as shown in the linked paper above in Fig. 2 (b1) and given here for brevity,
which one might expect if this was the process, especially since no acid washing process was done in an attempt to remove metals.
What is also interesting is that, of the diamonds that remain in LION 2 - they seam to have been wholly or in part, turned 'black' could this mean they have turned into graphite structure? Have the LION 2 diamonds been fully converted to graphite? The process in the paper is a surface effect.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
3 comments:
a) In the youtube video around 14 mins you mention "not massively convincing" and I would concur. Especially reactions involving multiple 13C atoms seem unlikely as the abundance of 13C is only 1% making that highly unlikely.
b) About melting points. Yes, I agree the contaminants aren't likely to come from the alumina tube, but the argument of the melting points alone might not be sufficient. Elements like sodium, magnesium and barium can have quite a strong melting point lowering effect when added to the mix. Of course you need a bit of concentration for this effect to take place. Key term here is 'ceramic flux'. It won't apply if the concentration is below 5%.
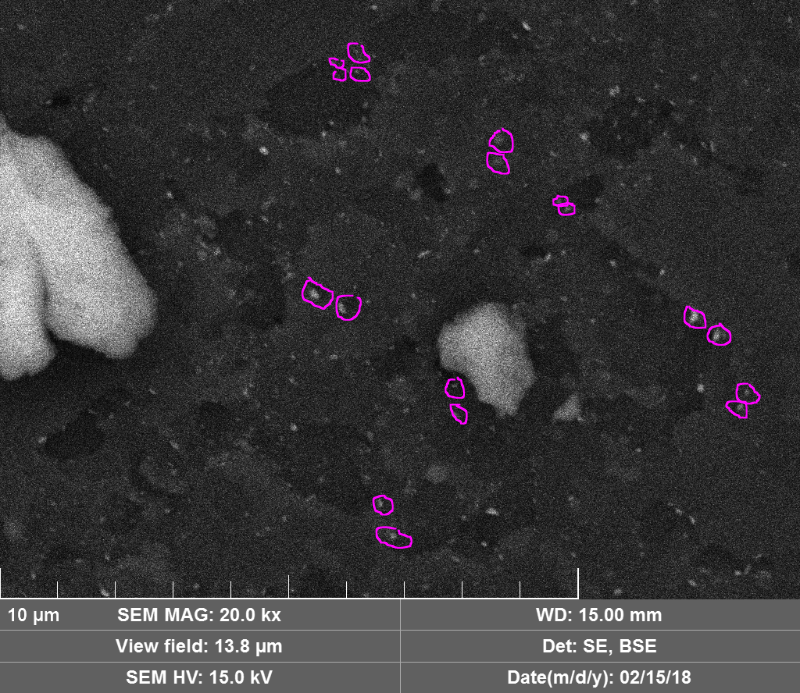
c) Wow, the images of the damage to the diamond discs is spectacular! I'd like to point out that in an earlier youtube video (https://www.youtube.com/watch?time_continue=8&v=BhitBhess2E) you pointed out the double spots with similar size (and shape?). It looks like that's going on here too:
I've just marked a few on the back scatter image, but the regular image has them too. They seem to be all over the place in fact! It would be interesting to compare the elemental composition between different locations on the virtual line connecting the spots. The same comparison between linked spots might help determine if material has traveled from one spot to another. Thanks for this post.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
a) Firstly the Ti was between 0.3% with low certainty. Secondly the Ti can from using the George Ohsawa reaction:
28,30Si + 18O > 46,48 Ti
Additional 13C can be bred via CNO reaction path ( addition of 2 protons to 12C + decays ) - See here
b) There was a mention that some chemical process might be in play, what you contribute is good detail - especially since in most places these other elements are 0.8% or below with spots around 2%
c) not so convinced on the double spots here since there does not appear to be the symmetry - however, it may manifest differently in the dynamic process of 'mining' the diamonds - or it may be that what we are seeing here is just the cooled / solidified residue of the material that was acted on, physically wetted to the cut plane at the end of the action, resulting in asymmetry. You are right in saying that analysis of the compositions would shed more light on potential relationships. This is certainly something to explore when taking another look at the samples.
Thanks for your skilled contribution.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
"Additional 13C can be bred via CNO reaction path ( addition of 2 protons to 12C + decays ) - See here"
That seems unlikely: Since the pads are soaked in heavy water it would be a deuterium environment rather than a hydrogen environment. There's some H in the D2O, but would that be enough? The CNO cycle wouldn't run on D as the first step in the cycle would produce stable 14N if that would in fact be the fusion path. Similarly 14N with D would produce stable 16O.
As for the symmetry in the double spots. We have a difference of opinion/observation/belief there. I guess some algorithm would be better at recognising symmetry without expectations and could supply a degree of certainty along with the observation. Bare in mind the 'entry' and 'exit' spots could be different looking: if this phenomenon is analog to solar flares for instance, the symmetry would be in the magnetic field lines rather than the plasma that roughly follows the pattern. Macro symmetry with localised asymmetry.
It would be awesome to see the diamond lite up with rainbow shaped 'electron flares' (if that is what happens). What would that even look like? And how would the magnetic field generated by the heater coils interact with these dynamic structures? Would it bend the donut shape? Also, why would the structure curve back to the surface to form a half a donut shape?
Looking forward to seeing more!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Granted it was only 0.2% H2O in the Merck D2O.
You need to collate the form of Strange Radiation track 'paw prints' in LION with the Surface features.
There will be more.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit