If we have ever had the opportunity to observe the mercury that specks the floor of a hospital after having broken a thermometer of the ancients, we will realize that the balls of the metal remain immobile splashing the floor of the room with silver. However, if what we drop is a glass of water, it spreads all over the ground, moistening whatever is within reach. To clean the water we will need an absorbent surface that covers the whole puddle and soaks the better. To clean the mercury from the thermometer, you only need a small sheet of paper to push the balls and see how they gobble each other until you complete a single ball that can be picked up by the same sheet of paper. And nobody will ever know that a mercury thermometer broke there.

Source
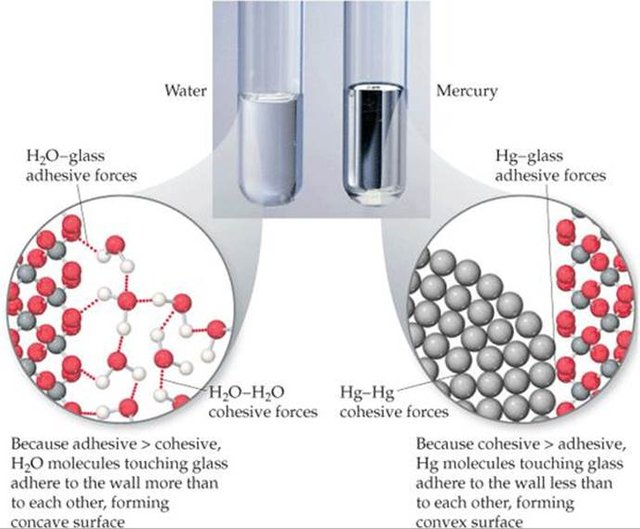
Both substances are liquid; however, the fact of sharing status does not make them sisters and their behaviors are far from similar. Mercury is a metal that has true avidity for itself, we could say that it is an egotistical element that likes its own existence. Water is a non-metallic, polar covalent compound that, contrary to mercury, is deeply altruistic and tends to relate to anyone who approaches it, hence it is the "universal solvent". The reason for so much mismatch is in their liaison forces.
Water, H2O, is a polar molecule. The intramolecular bonds are covalent and the intermolecular bonds are hydrogen bonds, the latter links much weaker than the covalent ones, which although exert a certain cohesion force between the molecules are not as strong as the metallic ones. The fact that the water molecule is polarized gives it the property of sociability because it wants to unite with any other related molecule (electrically speaking) that makes a hole in his life.
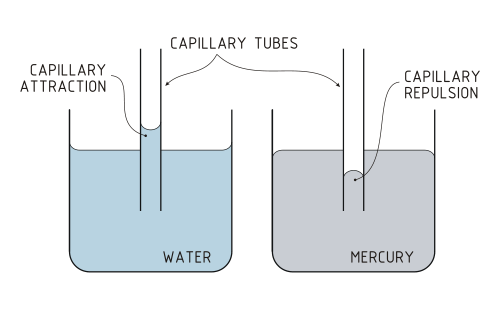
There is another phenomenon that shows how far apart both substances are, although both are liquid. Capillarity is a property of liquids caused by surface tension, by which they can ascend or descend through a tube of small diameter or capillary whenever the liquid is touching both walls of the tube. If we were to compete in a climbing competition with mercury and water, we would see that water always wins.
Source
Again the molecular cohesion offers us the explanation for this physical phenomenon. In water, the force that is established between the molecules that touch the walls of the capillary is greater than the cohesion force between the molecules that are on the surface, so that when the force of gravity pulls all of them, an depression in those who have the weakest forces; that is, in the molecules on the surface that are in the center farthest from the walls and a concavity is created. On the other hand, in the case of mercury, the cohesive force of its molecules is much greater than that of the molecules with the walls of the capillary and when gravity exerts its effect it depresses the weakest zone, that of contact with the walls, creating a convex profile. In fact, if we submerge a capillary in water it rises by the same to the mercy of the intense relationship of the water molecules with those of the glass, whereas if we submerge a capillary in mercury it descends below the level of the external liquid attracted by the molecular forces of its metal bonds.
Source
So, if you ever want to make a liquid ascend through a capillary, do not count on mercury. Liquids yes, but not cousins.
References
I hope you liked it, and that it will be useful, thank you for your time, do not forget to comment and, if you like, vote and follow me for more material like this. @noirac

This is a test comment, notify @kryzsec on discord if there are any errors please.
Being A SteemStem Member
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit