Nuclear batteries derive usable energy from radioactive decay. They are extremely useful in the harshest environments if power is needed. Today I'll be going over how direct-charge batteries, the simplest type of nuclear battery, work, including several design complications and why it's very difficult to reproduce this simple design in a DIY setting.
Remember that nuclear batteries are not nuclear reactors. Nuclear reactors induce nuclear reactions whereas nuclear batteries simply generate energy from naturally occurring radioactive decay (although the decaying element is usually not naturally occurring).
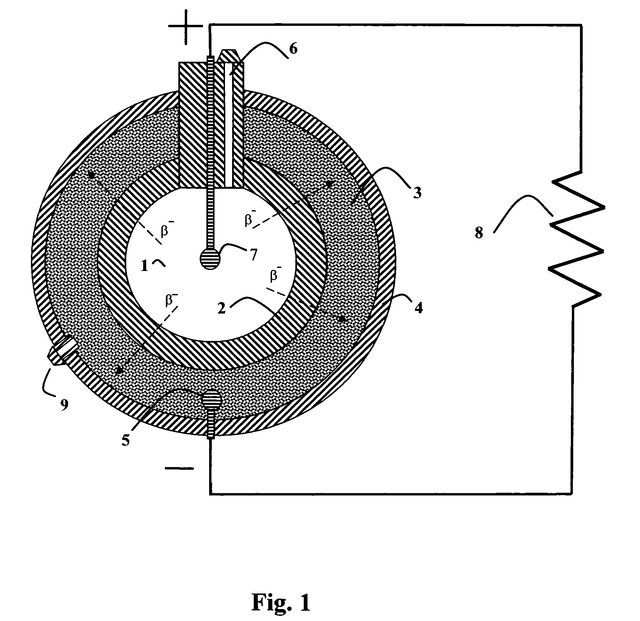
Schematic of a type of direct-charge battery
Image credit
Radioactivity
Radioactive decay releases several types of high energy particles: Electrons, Helium nuclei, neutrons, photons, neutrinos, positrons, and more. Many of these particles carry an electric charge - the most common being alpha particles (+2, Helium-4, containing 2 protons and 2 neutrons) and electrons (-1). Many different isotopes are radioactive, including most very heavy elements.
Photons, neutrons, and neutrinos make up the types of radioactive decay products that aren't charged. Neutrinos stand out in that they are nearly impossible to detect anyway. The direct-charge nuclear battery, as you will see, relies on charge, so these three particles are not useful to us in this case.
To avoid confusion, from here on out I will refer to fast-moving electrons as beta radiation and fast-moving Helium-4 nuclei as alpha radiation. This terminology is pretty old but also quite convenient so I don't have to keep saying Helium-4.
Direct-Charge Batteries: Charging Capacitors with Radiation
Direct-charge nuclear batteries are nothing more than self-charging capacitors. In an ordinary capacitor, an external voltage applied across the capacitor causes charges to gather on the parallel plates on the capacitor. This produces an electric field across the capacitor and a voltage difference across the output leads according to its capacitance and charge.
Capacitors always involve separate conductive plates or foils separated by either a vacuum, air, or a dielectric material. What this means is that the two metal plates collecting charge are always separated by something that isn't conductive - charge typically can't cross the gap to neutralize the oppositely charged plate.
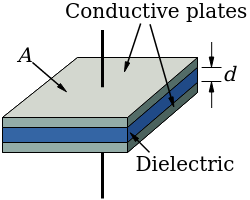
Parallel-Plate Capacitor
Image credit
But if I just make a capacitor and leave it on the ground, with no battery or voltage source attached, nothing happens. The capacitor sits there, with no charge on the plates, and no voltage across the capacitor leads. It will stay this way... unless we add one new part.
Say we put a radioactive source on one of the plates. Say this radioactive source is a beta emitter - it decays via beta decay, producing electrons (beta radiation) and electron antineutrinos. Beta radiation is generally easy to absorb in metals, so the electrons emitted out the back of the source will simply become embedded in the metal plate and eventually recombine with a charged ion - no net charge gained. But the electrons that fly out across the gap between the capacitor plates will produce interesting results.
An electron that is produced via a decay reaction on the first plate can cross the capacitor gap and reach the second, also uncharged plate. However, remember that ordinary charge cannot cross this gap - the beta electron is only able to do so because of its extremely high kinetic energy. This beta particle strikes the second metal plate and rapidly slows to thermal speeds. But once again, now this electron can't cross the gap - and so the second plate has a gained a net charge of -1 e. This in turn induces an equal and opposite charge of +1 e on the original plate, with the electric field crossing the gap. Now there is net charge Q on the capacitor, and as such, voltage across the plates. We have charged up this capacitor using nothing but a single radioactive decay - and where there is charge on a capacitor, there is energy on a capacitor.
What that single electron crossing the dielectric gap did was add a tiny amount of energy to our "battery". If you wanted to, you could now hook up a resistor across the capacitor and the tiny 1 e of charge would flow back to the other plate, neutralize it, and deposit a tiny amount of heat energy in the resistor.
What if our radioactive source is very active, with many decays per second? Now many electrons cross the gap every second. Only electrons that reach the second plate contribute to the overall charge on the capacitor, so the net charge on the second plate continue to build up. The voltage continues to increase. We continue to gain energy on our battery!
Neglecting any outside effects, this process continues for a long time. Electrons keep crossing the gap with their very high initial kinetic energies. Charges keep building up and the voltage rises and rises. But this must stop at some point.
Let's say the radioactive source is emitting electrons right out of the first capacitor plate with a decay energy of 100 keV (kiloelectronvolts). Since electrons have a charge of -1 e, an electron with energy of 1 electronvolt will be totally stopped if it crosses a voltage/potential difference of 1 volt. This means that the voltage on our nuclear battery will continue to rise until the voltage reaches the maximum energy of the beta decay for the radioactive source.
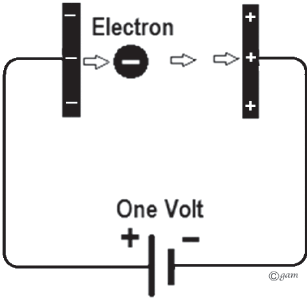
This electron will hit the positive plate with a kinetic energy of 1 electronvolt. Likewise, if the electron enters the gap coming from the opposite direction with an energy of 1 electronvolt, it will reach the negative plate with no energy.
Image credit
Beta decay is a three-body process, so the electron doesn't come out with the same energy every time - it has a spectrum of possible kinetic energies, with an average and maximum value. As the capacitor charges, electrons will be slightly slowed down when they cross the gap due to the build-up of like (negative) charge on the second plate. At first, almost every electron emitted in the direction of the second plate crosses the gap - only the few unlucky electrons that were released with very little kinetic energy (this means that the antineutrino stole most of the decay energy, never to be seen again) are stopped by the increasing electric field across the capacitor.
As the voltage rises, the electric field strength will rise, and the electrons will reach the 2nd plate with less and less energy. Finally, once the voltage equals the electronvolt value of the maximum kinetic energy of any of the beta radiation, the capacitor will be fully charged and no further energy will be built up in it, because the fast electrons are physically unable to breach the massive electric field and reach the second plate (they are literally flung back into the first plate!). In the case of the 100 keV beta decay, the capacitor will stop charging once it reaches 100 kilovolts (100,000 volts!!).

Strontium-90 is a decent energy beta emitter and a good candidate for direct-charge battery applications.
Image credit
Unique Aspects and Complications
The direct-charge nuclear battery has some weird properties.
Being a capacitor, at voltages much lower than the maximum voltage the efficiency of the battery actually increases as the charge increases. This is because (at voltages low enough that essentially none of the electrons are stopped before they reach the second plate) the charge across the capacitor increases at a linear rate (constant radioactive decay rate means constant build-up of charge), causing the capacitor voltage to also rise at a linear rate. But, since the stored up energy of the capacitor is proportional to the square of voltage, this means that our nuclear battery's efficiency rapidly rises as the voltage rises. Charging the capacitor from 0 to 1 volt will take the same amount of time as charging it from 3 to 4 volts but will generate far less energy. Because of this, I've noticed that if you wanted to quickly reach maximum power output it would actually be better to initially charge up the capacitor to a higher voltage and then start to extract energy.
Another curious feature of these batteries is that initially, the energy of the radiation produced has nothing to do with the energy generation rate of the battery. Two radiation sources, each producing 1000 decays per second, will both initially charge up a direct-charge battery at the same rate (and produce the same power output) even if one of the sources has beta radiation with 100 times the energy of the other source. The difference is that the higher energy source will be able to charge up the capacitor to a much higher voltage, and will eventually reach higher power rates.
These features make the direct-charge nuclear battery somewhat impractical: The initial energy production rate is very low regardless of radiation energy, and in order to utilize the battery to its full potential you have to let it charge up to high voltages. And these voltages can get stupidly high: For example, a battery built out of Strontium-90 wouldn't fully cut-off the flow of charge until it reached a capacitor voltage of 546000 Volts, as the maximum decay energy is 546 keV. This would produce such a high electric field across the capacitor that it would be extremely difficult to actually create: With an air or dielectric gap, an electrical arc would form, while in a vacuum, electrons would start tunneling out of the second plate and crossing the gap long before this voltage was reached.
Of course, there are also a few benefits. The direct-charge nuclear battery is probably one of the least likely nuclear batteries to fail if the voltage is kept in check. There are no parts to fail and the system is robust: The radiation source will decay regardless of anything, and the metal plates will not be damaged by the radiation environment. Some other nuclear batteries such as betavoltaics suffer from radiation degradation relatively quickly.
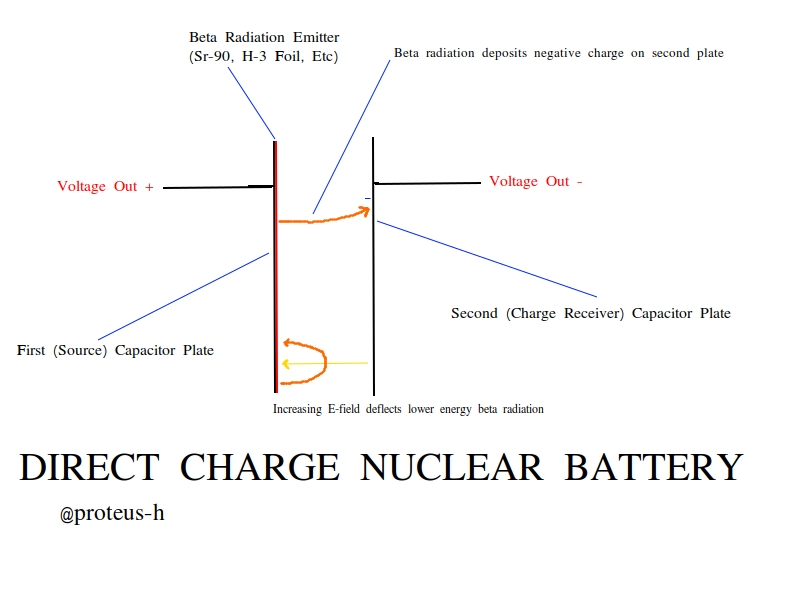
More setbacks, and why it's really hard to do this yourself - My Experience
So if this works so well, this should work even better for alpha radiation. After all, alpha particles have twice the charge of beta radiation (+2 vs -1) and typically have far higher energy than most beta decays (Americium-241 produces 5.5 MeV alpha radiation). Then it should be extremely simple to build a direct-charge battery yourself: Simply extract Am-241 from a smoke alarm and put some aluminum foil in front of it. Charge will build up and you can generate energy!
Except you can't. I speak from experience: What I just said is wrong and doesn't work in practice.
What I believe is the issue is that alpha particles are heavy particles compared to electrons. Alpha radiation ionizes air extremely easily. That's why it's used in smoke alarms! Thus if you try to build a direct-charge nuclear battery using an alpha source (like Am-241) with an air gap, the alpha particles will ionize so much air that charge can easily flow back across the gap and neutralize the plates: You generate no voltage build-up and no energy, and are left sad and irritated like I was when I tried this the first time before realizing this.
You could put a denser material in between the capacitor plates (a dielectric) to lessen the ionization losses. This works well for beta radiation based on the papers I've read. But alpha radiation is stupidly easy to stop and won't make it past any barrier thicker than a few dozen microns, even if that barrier is styrofoam. So that doesn't work well for alpha emitters.
Okay, so just use a beta emitter, you say. If alpha radiation is terrible for this, why not use beta radiation? The problem here is that it's very hard to find strong pure beta emitters without emptying your wallet to pay for professional sources. Alpha radiation is great because Am-241 sources are extremely easy to obtain legally and cheaply, whereas for beta radiation you would have to settle for relatively small sources like the pseudoscience patches or spread-out sources like uranium ore. You need a lot of charge movement to get detectable voltages across capacitors, so only more concentrated radiation sources will do here.
The only solution I've come up with is to just build your nuclear battery in a vacuum: But then you have to run a vacuum pump that is using millions of times more energy than your battery can produce and you ruin the point. Unless you can keep a permanent vacuum seal!
On that note, if you have any idea on how to make this work with legal, cheap radiation sources in a DIY setting, let me know. I've exhausted most of my ideas of trying to avoid using a vacuum. I've tried and failed to make this seemingly super simple device on my own many times, but hopefully I'll eventually get it right. Or it could just be that I am miscalculating what kind of charges would actually be detectable and I'll never be able to do it with a legal/safe radiation source - who knows? :)
Direct-charge batteries have an important place in history as the first ever nuclear battery, first produced 100 years ago by genius Henry Moseley. However, due to the setbacks mentioned above, they aren't often used or built, losing out to semiconductor and thermoelectric based designs. Nuclear batteries are still used on things like deep space spacecraft, but typically the type used utilizes waste heat from powerful alpha emitters to generate decent amounts of electricity without the high voltages and finicky efficiency of direct-charge batteries.
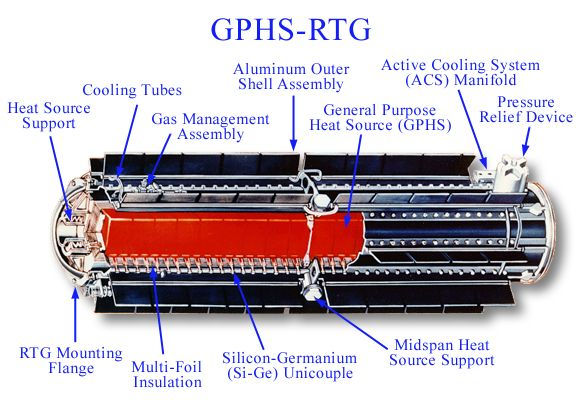
This is a Radioisotope Thermoelectric Generator, or RTG. These are much better nuclear batteries, but are huge, expensive, and use very rare materials (Pu-238). These devices are used to power remote lighthouses and spacecraft travelling on the edge of the solar system.
Image credit
I hope you learned something from this. Direct-charge nuclear batteries are pretty simple but I still find them interesting ... and frustrating, because after a lot of time trying I still haven't been able to build one.
What I like about nuclear batteries is that they can generate power from the violent nuclear reactions of decaying elements without relying on a giant potentially dangerous nuclear reactor.
If you have any comments, questions, feedback, or corrections, do not hesitate to let me know.
Thanks for reading!
Additional Sources:
1 - Wikipedia Nuclear Batteries
2 - Atomic Batteries: Energy from Radioactivity
3 - Henry Moseley - Biography
4 - History of Direct Charge Nuclear Batteries
5 - Radioisotope Thin Films for Microsystems
Interesting post!
Improve the quality of your posts.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
You wrote:
Do you have any helpful suggestions @syarol how @proteus-h can improve the quality of his posts? He offers a lot of information in this post and many of his other posts, so it would be helpful to offer some tips on how he can improve, if you have those tips. Thanks.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I'm glad you found the post interesting! Please let me know any corrections/criticism you have so that I can improve.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit