Detectors of separated chemical products in chromatography
During the development of gas chromatography, dozens of detectors have been investigated and used. The most frequently used are described in the sections that follow.
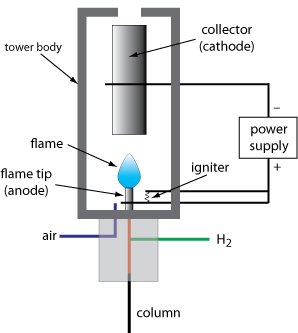
* Flame ionization detector (FID):
In gas chromatography, the flame ionization detector (FID) is one of the most used detectors and, in general, one of the most possible. In a burner, the effluent from the column is mixed with the air and then electrically ignited.
The great part of the organic compounds, at the moment when it is pyrolyzed at the temperature of a hydrogen flame, originates in the electrons that reach the electricity through the flame. Between one end of the burner and the collector electrode that is located above the flame, a difference of hundreds of volts is used, and for the measurement of the resulting current (around 10 ^ -12 A) a high impedance amplifier is used operationally.
The ionization in the flame of the compounds that are not well accepted as the process is not visualized the number of atoms that have turned into carbon atoms converted into the flame. The flame ionization detector, because it is a detector that indicates the number of carbon atoms entering the detector per unit of time, is a mass-sensitive detector, instead of a system sensitive to concentration. Thus, this detector has the advantage that changes in the flow velocity of the mobile phase have little effect on the detector response.
source
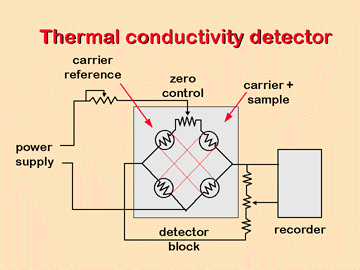
• Thermal conductivity detector (TCD):
This detector is based on the various changes in the thermal conductivity of the gas stream produced by the existence of analyte molecules. This module is sometimes called a katharometer. The katharometer sensor is in an electrically heated element where the temperature, at a constant electrical power, depends on the thermal conductivity of the surrounding gas. The heated element may be a thin strand of platinum, gold or tungsten. The resistance of the cable or the thermistor gives a measure of the thermal conductivity of the gas.
For the configuration of the detector components, two pairs of elements are used, one of the pairs is placed in the effluent flow of the column, and the other in the gas stream prior to the sample injection chamber. Alternatively, the gas stream can be divided into two streams one of which flows through the injector and the other does not. In any case, the effect of the thermal conductivity of the carrier gas is compensated, and the effects of the variation of flow, pressure and electric power are minimized.
The thermal conductivities of helium and hydrogen are approximately six to ten times greater than those of most organic compounds, so that, even in the presence of small amounts of organic matter, a relatively large decrease in thermal conductivity occurs. of the column effluent and, consequently, the detector experiences a marked increase in temperature. The conductivities of the other carrier gases are more similar to those of the organic constituents and for this reason with a thermal conductivity detector, hydrogen or helium should be used as the carrier gas.
source
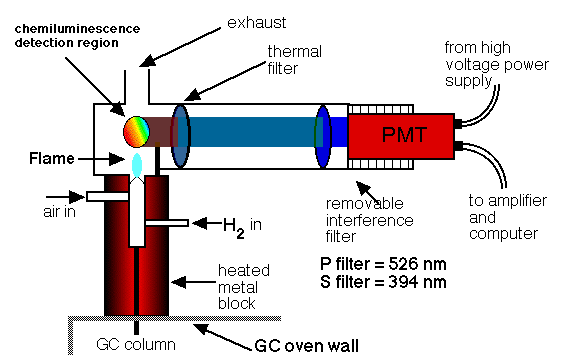
• Thermionic flame detector (FTD):
The thermionic detector (FTD) is a detector that selects organic compounds that include phosphorus and nitrogen. Its response is 10 times more than that of a nitrogen atom and 10 ^ 4 to 10 ^ 6 times greater than that of a carbon atom with respect to a phosphorus atom. Compared to the flame ionization detector, the thermionic detector is 500 times more sensitive for phosphorus-containing compounds and approximately 50 times more sensitive to nitrogen samples.
These properties make thermionic detection a particularly useful system for the detection and determination of many phosphorus-containing pesticides. A thermionic detector has a configuration similar to the flame detector. The effluent from the column is mixed with hydrogen, passes through the flame, and burns. The hot gas flows around an electrically heated rubidium silica ball, which is maintained at about 180 V with respect to the collector. The hot ball forms a plasma that reaches a temperature of 600 to 800 ° C. Exactly what happens in the plasma, which causes a large number of ions to be produced unusually from molecules containing phosphorus or nitrogen, is not really well established, but the result is a large current of ions, which is used for the determination of compounds that contain these two elements.
source
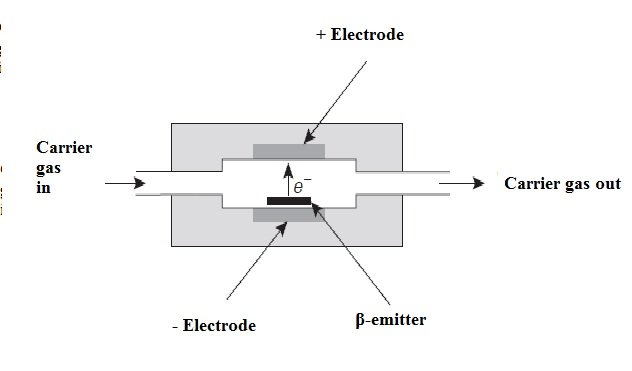
• Electron capture detector (ECD):
The electron capture detector (ECD) operates in almost the same way as a proportional meter for the measurement of X radiation. In this case, the effluent from the column passes over a β (beta) emitter, such as nickel-63 or tritium (adsorbed on a sheet of platinum or titanium). An electron of the emitter causes the ionization of the carrier gas (often it is nitrogen) and the production of a burst of electrons. From this ionization process, in the absence of organic species, a constant current between a pair of electrodes results. However, the current decreases in the presence of organic molecules that tend to capture electrons. The response is not linear unless the potential of the detector is applied in the form of pulses.
The electron capture detector is a distinctive proposition, being very perceptive to molecules that contain electronegative functional groups such as halogens, peroxides, quinones and nitro groups; On the other hand, this detector is not sensitive to functional groups such as amines, hydrocarbons, and alcohols. It is important to note that this detector has an important application as is the determination and detection of chlorinated insecticides.
source
• Atomic emission detector (AED):
In this device, the eluent is introduced into a helium plasma obtained with microwaves, which is coupled to an emission spectrometer with series of diodes. The plasma is sufficiently energetic to atomize all the elements of a sample, to excite them, and thus obtain their characteristic emission spectra. These spectra are collected in a spectrometer that uses a series of diodes configured in a mobile plane, which is capable of detecting the radiation emitted from 170 to 780 nm. The series of movable diodes is capable of simultaneously controlling two to four elements in each position. So far, the data processing program supplied with the detector allows measuring the concentration of 15 elements. Presumably, a future software will also allow the detection of other elements.
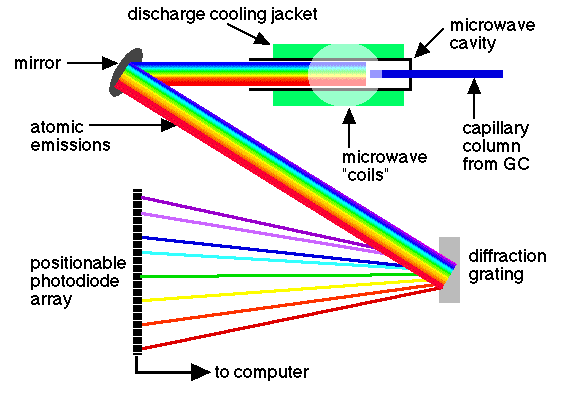
source
Amazing.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Amazing.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Amazing.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Not really familiar with but the post very informative. By the way, Please don't forget to follow our blogs and vote. Thank you.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit