There is a great interest in the electrodeposition of metals on organic films. This interesting is related to the metallization of organic materials, molecular electronics and the development of new nano/microfabrication techniques among others. As we Know the existence of defects in the SAMs limited their use as passive elements in MSM type devices. Therefore, in this post, we will study the electrodeposition of "Cu" on SAMs of different alkanethiols using defects in form positive:
For the confined growth of 'Cu' metal in nanoparticles.
To deposit "Cu" films of different thicknesses of which can be molded and ordered by patterns in the nano/microtechnology.
It is noteworthy that the use of SAMs to modify substrates in electrodeposition is limited by the electrochemical stability interval for their desorption.
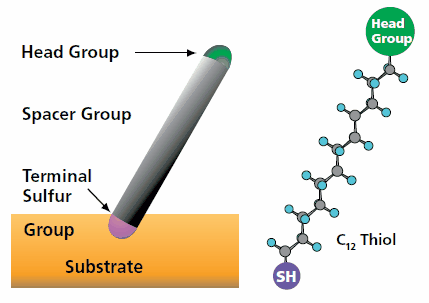
Experimental preparation of SAMs
For this experiment, I used gold as the metal substrate to perform the growth of confined structures of nanometric size, The "Au" receives a process of flaming with hydrogen flame, by means of which it is possible to orient its terraces of nanometric sizes in the plane
The alkanethiols were used in the growth of the nanoparticles (propanothiol C3 and hexanediol C6) and in the replication of micro/nanostructures (dodecanethiol C12)
The concentrations of SAMs vary according to the type of application in which they are used. In the case of the formation of the nanoparticles of "Cu" the concentrations were centered in a range between 5-7 mM, whereas in the case of the self-assembled ones for nanostructure replication, a 50 μM solution was used, both dissolved in ethanol.
The metal electrodeposition was carried out in a conventional three electrode electrochemical cell, where the working electrodes were micro and nanostructured surfaces, a platinum sheet as a counter electrode and a saturated calomel electrode (ECS) as a reference electrode.
In the case of electrochemical molding, the "Cu" was electrodeposited under electroplating conditions using a current density, "j", Which varied within a range of 1 to 10 mAcm-2. The working solution contained 0.6 M CuS O45H2O + 0.5 M H2S O4 + 0.025 mM thiourea at room temperature. In the case of the "Cu" nanoparticles, a deposition was made to sub potentials, where the solution used was 10-3 M CuS O4 + 0.1M H2S O4.
All the electrolytes were worn for 2 hours with ultrapure nitrogen, before carrying out any type of electrochemical test
Kinetic aspects of metal electrodeposition on substrates covered with SAMs
The process of metallic electrodeposition consists of two stages, the first is the so-called charge transfer, while the second is mass transfer.
Many published studies describe the phenomena and mechanisms involved in the transfer of cargo through SAMs.Porter and his 4 collaborators were pioneers in trying to explain the mechanism of charge transfer in electrodes modified with SAMs, through cyclic voltammetry. One of his conclusions was that the electronic transfer through the self-assemblies is carried out by means of two mechanisms: tunnel effect or through the defects of the monolayers. They suggested that the faradaic current could be separated into three distributions. At low overpotentials, charge transfer occurs directly in the bare areas of the electrode surface. A second contribution to the total current is the load transfer by tunnel effect in the areas where the SAM is partially collapsed (boundary of the domain, folding of chains in edges of steps of the Au (111)
In these cases, the tunnel current varies exponentially with the thickness of the molecular film. Finally, the third contribution is the load transfer by tunnel effect through the regions of the monolayer free of defects. Another study is carried out by Chidsey, where they analyze the load transfer by tunnel effect in monolayers but with an electroactive terminal functional group. The results about the dependence of charge transfer kinetics with the "donor-acceptor" distance using alkanethiols of different chain lengths demonstrated that the contribution by tunnel effect becomes important when the donor-acceptor distance is less than the nanometer; say a chain of the order of 3 to 4 methyl groups. Above this distance, the charge transfer becomes negligible in the monolayer regions free from defects. Then charge transfer only becomes predominant in defective areas of the molecular self-assembly, where clearly the thickness has been drastically reduced. From the above, we could conclude that the process of electrodeposition on electrodes modified with long chain alkanethiols load transfer would only be carried out in the defects of the monolayer.
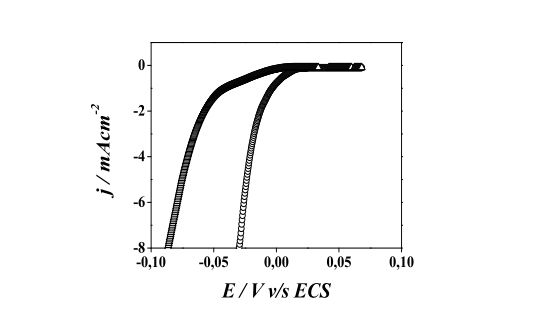
In the case of "Cu" electrodeposition, the current density profiles, "(j)", and potential, E, are very different for "Au" electrodes with and without dodecanethiol coverage. These profiles show that the alkanethiols on gold strongly hinder the electrodeposition of "Cu" since to obtain the same current density "(j)" is needed to work at more negative potentials than those used to deposit "Cu" in the absence of the SAMs. In concentrated solutions, such as those used to deposit massive "Cu" (OPD) in this work, where the control of the electrochemical process is given by the load transfer, the overpotential introduced by the SAM can be related to the difficulty of the electronic transfer to through the hydrocarbon chains. Also, the number of "Au" sites available for "Cu" nucleation in a surface covered by a SAM is much smaller than that which is present in a substrate of "Au" discovered. Therefore, in the presence of SAM, the nucleation rate is markedly reduced. In addition to forming "Cu" nuclei at Au sites, "Cu" ions will diffuse and/or migrate through a highly hydrophobic medium. The presence of overpotentials caused by the SAMs is important and should be considered since in galvanic conditions, potential values can be reached to which the thiol is desorbed by reductive desorption.
I'll keep talking about this experiment in the second part this was a large experiment to explain it in one single post,
I hope you like my research so far
Thanks I'm @rossybellp (:
For more references about it you can check the following books:
Azzaroni O, Schilardi P L, and Salvarezza R C. Electrochim. Acta, 48:3107, 2003.
Finlklea H O. Langmuir, 3:409, 1987.
Chidsey C E. Science, 251:919, 1991.
Porter D M, Brigth T B, Allara D L, and Chidsey C E. J. Am. Chem. Soc., 109:3559, 1987.