Now try friends imagine these objects consist of only a few molecules arranged in such a way as to be able to perform movements like those objects. At first, it may sound impossible to synthesize it.
The development of computing demonstrates how the miniaturisation of technology can lead to a revolution. The 2016 Nobel Laureates in Chemistry have miniaturised machines and taken chemistry to a new dimension. Jean-Pierre Sauvage, born 1944 in Paris, France. Ph.D. 1971 from the University of Strasbourg, France. Professor Emeritus at the University of Strasbourg and Director of Research Emeritus at the National Center for Scientific Research (CNRS), France. Sir Fraser Stoddart, born 1942 in Edinburgh, UK. Ph.D. 1966 from Edinburgh University, UK. Board of Trustees Professor of Chemistry at Northwestern University, Evanston, IL, USA. Bernard L. Feringa, born 1951 in Barger-Compascuum, the Netherlands. Ph.D.1978 from the University of Groningen, the Netherlands. Professor in Organic Chemistry at the University of Groningen, the Netherlands. Nobel source
However, the discovery that broke the impossibility that was ultimately rewarded with the 2016 Nobel Chemistry award. Jean-Pierre Sauvage, Sir J. Fraser Stoddart, and Bernard L. Feringa, each from France, Scotland and the Netherlands, were awarded the most prestigious award in the world of science for their contributions to designing and synthesizing molecular machines.
Starting from a molecular chain
If friends have studied organic chemistry, what is usually imagined is a series of long aromatic rings or carbon chains with several oxygens or nitrogen atoms tucked between the rings and chains. These organic molecules can then interact with other molecules through intermolecular forces and can also bind to form larger molecules with covalent bonds.
In the middle of the 20th century, chemical scientists tried to make these molecules not "bond" with other molecules, but they were strongly linked to each other. In other words, one molecule is locked with another molecule with a mechanical bond without the atoms of each molecule forming a covalent bond with atoms from another molecule. This is what is considered as the forerunner of molecular machine synthesis: molecular chains.
Over time, many publications reported that they had successfully synthesized molecular chains. However, in reality the final compounds reported to contain the molecular chain are very few and the manufacturing method is very complicated.
Until finally in 1983, a professor from the University of Strasbourg, Jean-Pierre Sauvage and his research team managed to synthesize a compound consisting of two large ring molecules adrift with each other called Catenane. With the help of copper ions, Sauvage and his team achieved a final result of 42%. A very drastic surge from what was previously synthesized.
Sauvage's scientific work has focused on creating molecules that mimic the functions of machines by changing their conformation in response to an external signal. His Nobel Prize work was done in 1983, when he was the first to synthesize a catenane, a complex of two interlocking ring-shaped molecules, which were bonded mechanically rather than chemically. Because these two rings can move relative to each other, the Nobel Prize cited this as a vital initial effort towards making molecular machine. The other two recipients of the prize followed up by later creating a rotaxane and a molecular rotor. Wikipedia source
Uniquely, the research conducted by Sauvage was originally not in the field of synthesis of organic compounds, but in the field of photochemistry. At that time he and his team developed complex compounds that could utilize energy from the sun to carry out chemical reactions. On one occasion he discovered that the molecules he made were similar to the molecular chains that other scientists were developing.
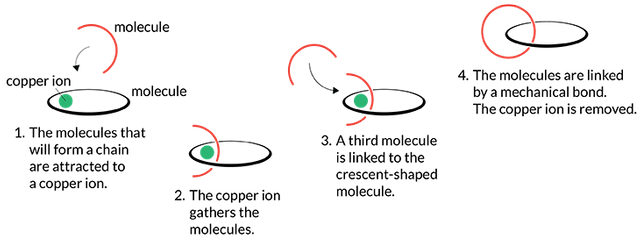
From there he and his team designed a reaction to make molecular chains. One ring-shaped molecule and a crescent-shaped molecule are linked around a copper ion, then another crescent-shaped molecule is reacted with a sickle molecule that has been linked to a ring molecule around the copper atom. The copper ion is then removed so that only two ring-shaped molecules are interconnected with each other.
Before Jean-Pierre Sauvage succeeded in synthesizing Catenane, research to make molecular chains was seen as research based solely on curiosity and not functional chemistry. It was only after the success of Sauvage and his team that research in this field was considered to have more potential in the future than just curious.
Sauvage itself after successfully synthesizing Catenane, continued his research by making one of the ring molecules evolve against other ring molecules when given energy.
Continue to molecular shuttle
Elsewhere, a professor from the United Kingdom, Sir James Frasser Stoddart and his team succeeded in synthesizing with the high efficiency of a molecular shuttle, ie one ring-shaped compound can move back and forth along a molecule that acts as an axis.
Actually the attempt to synthesize molecular shuttle which is then called Rotaxane is roughly starting with Catenane. But again at that time the efficiency was very low and the results were very little.
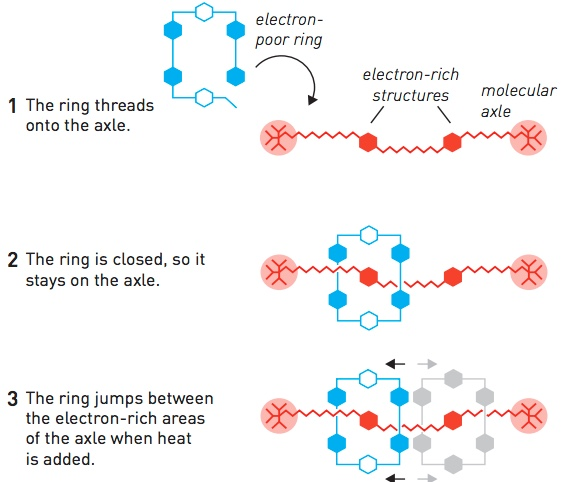
The Rotaxane synthesis method used by Stoddart and his team is to use the attraction between electron-rich and electron-poor molecules. The poor electron ring molecule which is initially open in one part is mixed with electron-rich axis molecules so that the ring molecules are attracted to the axis molecules.
Next, the open part of the ring molecule is closed so that the ring molecule can remain in its axis molecule. When given heat energy, the ring molecule moves back and forth between two electron-rich ends along the axis molecules
Not stopping there, Stoddart and the team continued to develop Catenane and Rotaxane and even collaborated with Sauvage in some of their research. Stoddart and his team continued the development of Rotaxane by modifying the molecules used so that the movement of the ring molecules back and forth can be controlled not only with heat energy but also by electrochemical redox and pH regulation.
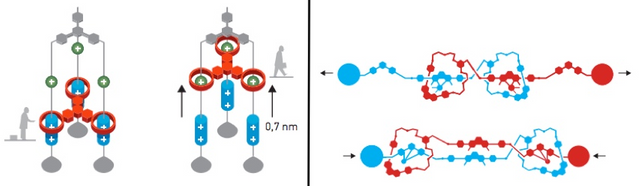
In addition, Sauvage and Stoddart also make other mechanical systems from Rotaxane. Stoddart and the team, for example, managed to make a molecular "lift" from a combination of several Rotaxane that can lift objects as far as 0.7 nm from the surface of their origin. Sauvage itself succeeded in synthesizing modified Rotaxane to resemble filaments in human muscles which can elongate and shorten with chemical induction.
Until molecular motors
Various studies in the field of molecular machinery systems by Sauvage and Stoddart attracted the attention of other scientists in this field. After rotaxane and catenane and their modifications were successfully synthesized, the scientists targeted to be able to synthesize motor molecules, a molecule that allows rotation in one particular direction after being energized. Finally, in 1999, a professor from the Netherlands, Bernard L. Feringa and his team managed to synthesize molecular motors.
Ben Feringa’s molecular motor (the first) was mechanically constructed to spin in a particular direction. His research group has optimized the motor so that it now spins at 12 million revolutions per second. Bernard Feringa was the first person to develop a molecular motor; in 1999 he got a molecular rotor blade to spin continually in the same direction. Ben Feringa’s four-wheel drive nanocar, with a molecular chassis and four motors that functioned as wheels. Using molecular motors, he has also rotated a glass cylinder that is 10,000 times bigger than the motor and also designed a nanocar. Nobel-prize-in-chemistry-2016 source
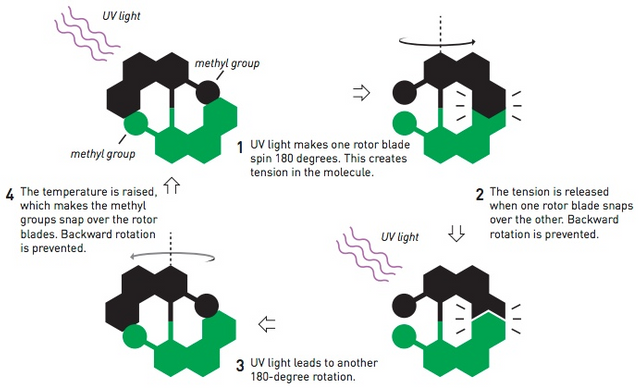
Unlike previous molecular motor research that uses more single bonds to allow rotation, Feringa and his team make molecular motors with rotation occurring in double bonds that can be isomerized.
They make two planar molecules bind to a double bond and add a methyl group to each molecule that functions as a buffer so that the reverse direction is not possible. This molecule can rotate in one direction like a motor when given light energy. In 2014, it was reported that the optimized molecular motorbike Feringa and his team had reached 12 million rounds per second.
Other machines
Until now, various other molecular machines have been made such as molecular propellers, molecular clips, to molecular switches with their respective functions. There are still many machines on a macro scale that have not been brought to this molecular level.
Going forward, the development in this field is expected to produce various types of nanoscale technologies that are useful for things like drug delivery, gene therapy, or even making molecular robots that can do various kinds of work. Interested in working in this field?
Reference:
Nobel-prize-in-chemistry-2016 source
Upvoted.
DISCLAIMER: Your post is upvoted based on curation algorithm configured to find good articles e.g. stories, arts, photography, health, community, etc. This is to reward you (authors) for sharing good content using the Steem platform especially newbies.
If you're a dolphin or whales, and wish not to be included in future selection, please let me know so I can exclude your account. And if you find the upvoted post is inappropriate, FLAG if you must. This will help a better selection of post.
Keep steeming good content.
@Shares - Curation Service
Posted using https://Steeming.com condenser site.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thank you
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations @sward! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPDownvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thank you so much
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit