Welcome to the post I of the topic about chemistry reactivity, you are reading a post that was elaborated thought of you. I invite you to make the most out of this work, since together we will embark on a journey through the wonderful world of chemistry. In this post, and those that I will share soon, you will get how the human being is able to build explanations around natural phenomena. You will have a greater approach in the understanding of formulas and chemical equations. Let's see!
Source
Each chemical transformation involves a change in the connectivity of the atoms. Some links are broken and new ones are formed. Organic chemistry is interested not only in the reactants and products of a reaction, but also in the details, especially the order of the breakage and bond formation processes. It is also interested in the study of reaction rates and investigates how the speed and products vary depending on the experimental conditions. Based on observations such as these, the details of the process or path that the reagents follow during their transformation into products are postulated. This reaction path is called the mechanism of the reaction. Below are some basic concepts that make up the chemical reactivity.
Formulas and stoichiometry
The chemical formulation allows the representation of the different substances and their composition. The chemical equations are used to represent chemical reactions.
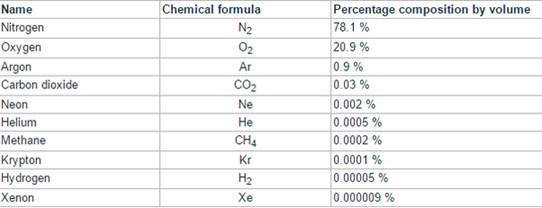
The chemical elements are represented by means of chemical symbols. The symbols are one or two letters that indicate the name of the element in Latin. The chemical compounds are indicated by chemical formulas in which appear the atoms that constitute them and some subscripts that indicate in what proportion they are. If there is a single atom of a compound element, the number 1 is not written.
Chemical formulas
It is an alphanumeric expression that is used to indicate the composition of a chemical substance and that is constructed from the symbols of its constituent elements, affected by numerical subscripts that report the number of atoms of each element that becomes part of it. the substance in question.
There are different types:
The empirical formula: indicates only the type and proportion of atoms that make up the molecule.
The molecular formula: indicates the type and exact number of each of the atoms that make up the molecule.
The two types of formulas tend to coincide for slightly complex substances, but they differ in substances with a high number of atoms. From the formula of a compound it is possible to easily determine its centesimal composition and, from the latter, it is also possible to know its formula.
Source
Chemical formulation
The chemical formulation consists in the representation of the elements that are part of a compound. In addition to the representation is the proportion of the elements that intervene as well as the number of atoms that make up the molecule.
To understand and learn to formulate it is very important to know the following concepts about the compounds:
Chemical element: a chemical element is a substance that can be broken down into other simpler substances, through a chemical reaction.
Chemical compound: it is a substance formed by the combination of two or more elements in fixed proportions.
Atom: it is the smallest part of an element that can form a chemical compound. According to the number of atoms that make up the molecule, these can be: diatonic (two atoms), triatomic (three atoms), tetrathonic (four atoms) or poly atomic (several atoms).
Molecule: is the smallest part of a chemical substance that can exist independently with its characteristic properties.
Oxidation number: the oxidation number indicates the valence with which an atom acts on a compound.
Valencia: the valence of an element indicates the capacity of combination of an atom.
The large amount of chemical substances that exist makes it inevitable that protocols are established to write the chemical formulas. If the substance is the result of the combination of two elements, it is written to the left of the electropositive element, while to the right is placed the element with the highest electronegativity. The subscripts must be chosen in such a way that the oxidation numbers multiplied by the number of atoms of each of the types compensate their sign and cancel.
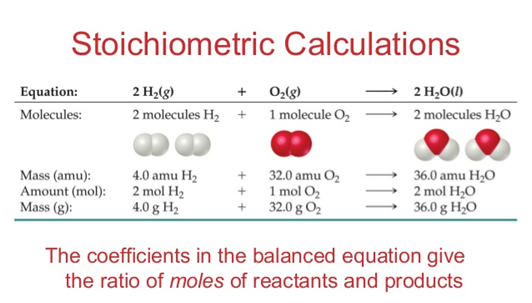
Stoichiometry
It is the part of chemistry that studies the quantitative relationships between the substances that intervene in a chemical reaction (reactants and products). These relationships can be:
mol-mol
mol-grams
grams-grams
mol-volume
volume-grams
volume-volume
Source
The relationships can be: between reagents and products, only between reagents or only between products. Any stoichiometric calculation that is carried out must be done based on a balanced chemical equation, to ensure that the result is correct.
The chemical equations
A chemical equation is a written representation that provides information about what has happened in chemical reactions. In order to understand the information that it provides, it is important that we know the characteristics and the parts that make it up. First, the participating substances are represented with respective chemical formulas ie: H2O CO2 NaCl C6H12O6 Likewise, it is important to indicate the physical state of the reagents and products, before continuing, do you know what a reagent is?, let me help you, well it is the matter with which you are going to experiment, in order to obtain or produce another one and that you can use it to carry out your daily activities, and a lot of the times you can also consume or sell. The definition of chemistry comes to my mind, where I am sure that it is the science that studies the matter, the structure, its composition as well as energy, but above all in the transformation into useful products for society. The physical state or state of aggregation of the participants is indicated with letters in parentheses on one side of the chemical formula of the substances. The letters used to indicate the physical state are the following.
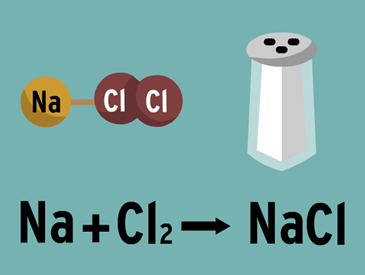
Source
| Physical State | Symbol |
|---|---|
| Solid | (s) |
| Liquid | (l) |
| Gas | (g) |
| Aqueous (dissolved in water) | (ac), (aq) |
Chemical bond
A chemical bond is the union between two or more atoms to form a higher order entity, such as a molecule or a crystalline structure. The compound that results from this bond is chemically and physically unique and different from its original atoms. The formation of links is always produced by a favorable balance of energy, that is to say, the linked atoms constitute a system of less energy than the atoms separately.
Types of chemical bond
Ionic bond. Due to the electrostatic attraction between ions. Typical of the combination of metallic elements with non-metallic elements.
Covalent link. Due to the sharing of electron pairs. Typical of the union between non-metallic elements.
Metal link. Due to the sharing of electrons collectively. Typical of metallic elements.
Greetings! Thanks for your attention.
For more information visit the following links.
- https://www.acs.org/content/acs/en/careers/college-to-career/chemistry-careers/formulation-chemistry.html
- https://www.khanacademy.org/science/biology/chemistry--of-life/chemical-bonds-and-reactions/a/chemical-bonds-article
- http://chemistry.tutorvista.com/physical-chemistry/stoichiometry-formulas.html
Thank you for this informative post. Even though I already know most things you mentioned it is still nice to have a good overview. Because in my case I mix up some chemical bonds sometimes. I hope the next parts are coming soon and it would be great if you maybe could tell me what the next posts will deal with exactly. Will you start with organical chemistry or maybe explain the different atom model? Keep up the good work
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit