Hello dear steemians, how are you guys . I will continue my post about #physics in real life #10 . Okay what I gonna to talk today is about thermal equilibrium . What is daily things that related with physics . So I hope you will continue reading okay . I will explain this using two method , which is Albert Einstein and Bloom's Taxonomy Method . So enjoy reading guys !
What is Thermal Equilibrium ?
First of all what is thermal equilibirum ?, thermal equlibrium is achieve when there are no heat flows between two physical things, in today cases the physical things are fish and ice ,water and ice .Systems in thermodynamic equilibrium are always in thermal equilibrium, but the converse is not always true. If the connection between the systems allows transfer of energy as heat but does not allow transfer of matter or transfer of energy as work, the two systems may reach thermal equilibrium without reaching thermodynamic equilibrium.
Relation of thermal equilibrium between two thermally connected bodies
The relation of thermal equilibrium is an instance of a contact equilibrium between two bodies, which means that it refers to transfer through a selectively permeable partition, the contact path. For the relation of thermal equilibrium, the contact path is permeable only to heat; it does not permit the passage of matter or work; it is called a diathermal connection. According to Lieb and Yngvason, the essential meaning of the relation of thermal equilibrium includes that it is reflexive and symmetric. It is not included in the essential meaning whether it is or is not transitive. After discussing the semantics of the definition, they postulate a substantial physical axiom, that they call the "zeroth law of thermodynamics", that thermal equilibrium is a transitive relation. They comment that the equivalence classes of systems so established are called isotherms.
Thermal equilibrium of a body in itself refers to the body when it is isolated. The background is that no heat enters or leaves it, and that it is allowed unlimited time to settle under its own intrinsic characteristics. When it is completely settled, so that macroscopic change is no longer detectable, it is in its own thermal equilibrium. It is not implied that it is necessarily in other kinds of internal equilibrium. For example, it is possible that a body might reach internal thermal equilibrium but not be in internal chemical equilibrium; glass is an example.
One may imagine an isolated system, initially not in its own state of internal thermal equilibrium. It could be subjected to a fictive thermodynamic operation of partition into two subsystems separated by nothing, no wall. One could then consider the possibility of transfers of energy as heat between the two subsystems. A long time after the fictive partition operation, the two subsystems will reach a practically stationary state, and so be in the relation of thermal equilibrium with each other. Such an adventure could be conducted in indefinitely many ways, with different fictive partitions. All of them will result in subsystems that could be shown to be in thermal equilibrium with each other, testing subsystems from different partitions. For this reason, an isolated system, initially not its own state of internal thermal equilibrium, but left for a long time, practically always will reach a final state which may be regarded as one of internal thermal equilibrium. Such a final state is one of spatial uniformity or homogeneity of temperature. The existence of such states is a basic postulate of classical thermodynamics.This postulate is sometimes, but not often, called the minus first law of thermodynamics.
Albert Einstein Method
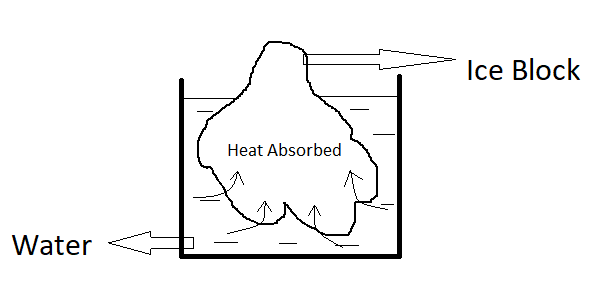
First of all , what you need to know , Ice has high latent heat of fusion . This will make the the ice block absorb more heat from the water . The increase of the latent heat of fusion , more heat can be absorbed by a substance .So , after the heat is absorbed by the ice block , the water tempreture will drop. It will cool down . The change of the tempreture is called as thermal equilibrium .
From the diagram , we can assume that the fish tempreture is higher than the ice tempreture .Ice and the fish are contact each other , so when it contact each other the process thermal equilibrium will occurred . The tempreture of fish will drop untill it is equal to the tempreture of ice .The fish contain more heat , then the ice will absorb the heat from the fish . Ice will start to melts as it absorbed the heat from fish .The bigger ice quantity ,the more heat will be absorbed by the ice .
.png)
Bloom’s Taxonomy Method
Since the water tempreture has high tempreture than ice , the heat is transferred from the water to the ice . This is because of the different of the tempreture between 2 object .
1.Comprehension(understanding diagram and make analysis)
In this method we need to follow the process of thinking skill, so this is the first process . We need to understand what the diagram show and make an analysis from the diagram .
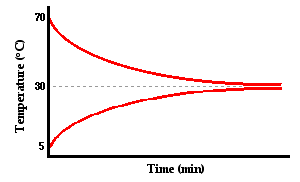
Okay guys , what we can see from the graph above is the water is lost heat when the ice gained heat . The water tempreture ,∅_w drop while the tempreture of ice ,∅_i increasing.
2.Application
The second step of this method is application, we need to apply our knowledge based on the diagram that we understand at the process before . So ,after a few minutes,the tempreture of the water and ice will reach a thermal equilibrium , 0’Celcius . At this point there is no net heat transferred .

3.Synthesis
This stage we need to apply physics concept into the real life application .
.png)
If the quantity of ice we put on the box bigger ,so more heat will be absorbed by the ice . One more things that we need to consider, it’s the size of the ice .Okay if the size of the ice smaller , it will makes the area of the ice will become bigger . If the area is increasing , the more heat will be absorbed .
4.Evaluation
For this step ,we need to think outside of the box because we need to design new things and make recommendation on how to make it more efficient . So what we need to do is ,we need to add foreign items such sugar or salt added into the ice . The additional of the foreign item will increase the attraction force between the ice molecules .The melting point of ice will increases . The ice take longer time to melt .

Summary
So what we get today is thermal equilibrium archived when there are no heat flow between each other connected physical system . We also know that the smaller the ice cube size , will make it surface area bigger so more heat absorbed . If the bigger mass of the ice, bigger heat will be absorbed by the ice.Then , if we add some foreign items such salt and sugar into ice cube ,it will make the ice tempreture to drop more or in another words it i will increase the melting point . 0'c to -ve somethings .Below is video about this topic , so I hope you will watch the video to make you more understand about this topic .
References
1.Wikipedia|Definition Of Thermal Equilibrium2.Image 13.Image 24.Youtube video5.Another Image I draw by myself
SteemSTEM
SteemSTEM , I am very proud with this community, because @steemstem is encouraging people to promote and write about Science,Technology,Engineering and Mathematics(STEM) postings on Steemit.Because this also an important things that we should know as our additional knowledge .
Join and learn about the project , Join us on steemit.chat:
(https://steemit.chat/channel/steemSTEM)

I hope you 'll find this useful. Upvote, Follow, Resteem.


Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
https://en.wikipedia.org/wiki/Thermal_equilibrium
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I have put this link on the article ..Thanks btw :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks for this post , now I am undertand wht is Thermal Equilibrium
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
My pleassure , really excited to hear that :), thanks for read my post
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit