Hi guys, it's being a while that I have last posted here, I must say it feels good to start writing back once again and hope you enjoy and learn from this one. With all that being said, you would realized from the title of this write up, a question based on my previous article "What it takes to choose the best battery for your solar power system"; an article that has some of its contents based on the conventional ideas I have acquired about renewable technology over time. In this article, i will be stretching beyond and wide across the horizon of my literal and basic understanding on battery storage system for renewable energy.
The increasing growth rate in the global use of generating electric power with renewable energy has greatly increase the demand for the development of a more efficient grid storage system. Although, there are various means of storing energy from these renewable sources but for smaller and smarter grids, batteries plays a crucial role and it is therefore essential to source for a more firm and sustainable type.
There are newly developed battery technologies out there for renewable energy but its seems like Li-ion technology has firmly established its stands as the best, due to its numerous applications in not just renewable energy but also portable electronic, smartphones, UPS, electric cars etc. and on top of that, it has a distinct advantages over all other technologies. But then I began to wonder if truly Li-ion is the best because if we are to take a closer look at some of its disadvantages such as cost and safety issues, there are other batteries out there that are more cost effective and of course with a more longer life span than Li-ion. Why I never bothered to mentioned them in the past is because I don't have enough facts then and on that note, stick with me on this one and find out some of discovery I have made.
When we talking about the latest battery technologies in town as far as renewable energy (solar and wind) is concern, we talk of salt water battery made by aquion energy, there is also the long forgotten nickel iron battery discovered during the days of Thomas Edison. From the little research I have carried out so far, I observed that one of these batteries has longer life span (nickel iron) while the other (salt water), longer depth of discharge than Li-ion batteries; quality features that are so much desired for every renewable battery bank. Although each of these technology has its own shortcomings that gives Li-ion edge over them.

Image source: Wikimedia under CC BY-SA 3.0
What if I tell you that there is a battery technology that has potential of relegating or if possible, make Li-ion obsolete; a battery with all the advantages but none of its disadvantages. Interesting right!
When i first came across this technology, it felt as if i have just discovered a gold mine and as a solar enthusiast, I so much desire to get to the basis of the matter. But before I proceed to uncovered this technology, I'll do a little introduction on how batteries are being charged and discharged as this will give us a better understanding of what am about to discuss.
I made mention in my previous article that battery do not actually store electricity but store it in another form of energy (chemical energy) in which we all are very familiar with. And that brings us to the two major types of batteries; Non-rechargeable and rechargeable battery. Both of them store and discharge electricity in the same manner but what differs is the numbers of charge and discharge cycles possessed by each types. Non-rechargeable, which also known as primary battery, can only be recharged once (when manufactured) and discharged once when used while rechargeable battery (secondary battery) have multiple charge cycles that allows them to be recharge and discharge over and over again until the charge cycle is completely exhausted.
Note that every battery is made up of tiny galvanic cells that are lined up in series to make up a greater voltage, with each individual cell voltage to be around 1.5V to 2.4V depending on the battery technology. Say for instance, a 12V battery with 2V galvanic cells, will have exactly 6 cells in it. For a better understanding of the discharging and charging process of a battery, we'll be make use of lead-acid galvanic cell and mind you, all battery technologies work on the same concept of electron flow.
Discharging process
The term "Galvanic cell" is no news as we are very familiar with this, even right from our high school days. But for the sake of simplicity, a galvanic cell (also known as a voltaic cell) is a typical type of electrochemical cell that undergoes electrons transfer using redox (oxidation-reduction) reaction to produce electric current. This so called transfer of electrons that take places occurs mainly between the anode and the cathode while the electrolyte is the medium through which electrons are being transfered. Taking a closer look at the internal structure of a lead-acid galvanic cell, its anode is a sponge-like lead plate, its cathode, a lead frame covered with lead dioxide while the electrolyte is a sulphuric acid
The conversion of chemical energy to electrical energy within the cell occurs gradually as each electrodes reacts with the electrolyte. The anode lead plate is firstly oxidized before it reacts with the electrolyte to liberate an electron and produces a lead suphate.
Likewise, the cathode is also reduced firstly by the protons from the electrolyte before reacting with it. This reaction yields lead suphate and water but instead of producing electron, the electron produced at the anode is used up at the cathode.
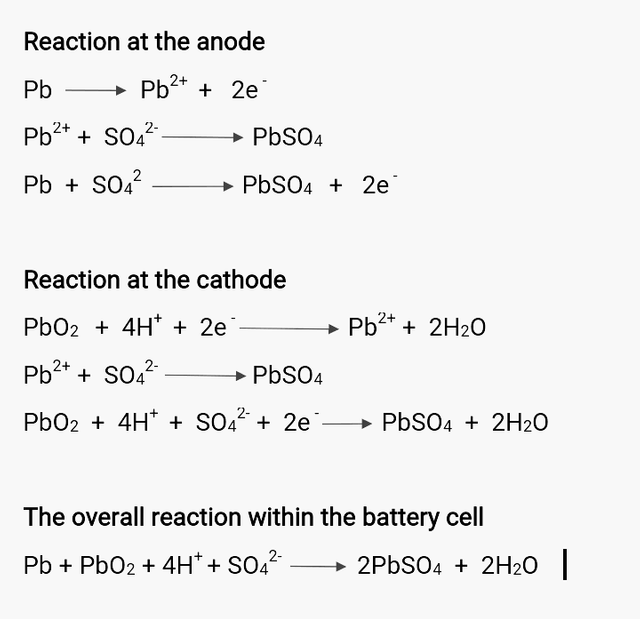
During the course of discharging, the lead suphate that is precipitated out is deposited on both electrodes while the hydrogen ion that used up at the cathode to produce water is gradually depleted and subsequently the concentration of the electrolyte. So to say in short, the energy stored in the cell is being used up
Recharging process
This is just the reverse case of the discharging process; a non-spontaneous electrolytic process that involves the introduction of a recharger (such as battery charger, solar or wind charger) that pumps electrons into the cathode of the battery. For the recharger to successful pump electrons into the cell, it must have a voltage that is slightly higher the cell voltage to drive the reaction in reverse direction, converting electrical energy to chemical energy. So to say in this process, the lead suphate on each electrodes is eliminated while electron is liberate at the cathode and used up at the anode, the exact opposite to that which occurs during discharging process.
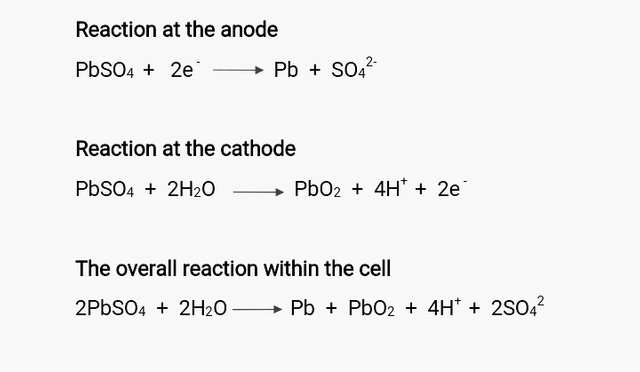
The end result of this action increases the concentration of the the hydrogen ion and electrolyte and in turn, the energy used up is replenished.
Am sure you are familiar with the saying that "Overcharging your battery will quickly reduces its life span and its energy storage capacity" but have you ever wondered what really happens within a battery cell when it is overcharged? The water within the cell electrolyzes causing the hydrogen and oxygen gas within the water to bubble. It is now this bubbling effect that degrades the electrodes within the cell forcing the lead suphate to fall off the electrode; an action that rapidly reduces the storage capacity of the cells.
And now, allow me to introduce to you the battery technology that threatens the reigns and dominance of Li-ion, Zinc air battery! A battery technology that is less dangerous, a more environmental friendly alternative to the widely known Li-ion technology. Why is Zn air batteries different from all other batteries? It belong to family of metal air batteries that has turned out over the time, a smart choice for a more green grid storage as a result of their high energy density and lesser environmental impact of its components. And of all the metal air batteries, Zn air is still the best of them all because of its availability and recycle potential.
This technology has been around for a while now, as the first ever non-rechargeable cell was manufactured by Thomas Edison century ago. Although he was unable to make them technologically and commercially viable as they were used then for railway signaling, navigation bouy and remote communication sites, researches carried out in the late 1980s indicates that this technology has the potential of competing with all other existing batteries in market then, owing to its high storage capacity and its safety like nature.
However, this expectation was cut short due to the problem of rechargeability; a problem that occur due to the formation of dendrite which eventually creates a short circuit between the electrodes during recharging. Even with this short coming, its non-rechargeable counterparts have found usefulness in several applications such as hearing aids, prosthesis and film cameras.
Zinc air cell is made up of a zinc anode, an alkaline potasium hydroxide electrolyte and an air cathode (carboneuous and porous in nature). The discharge and charging process is similar to that of lead-acid cell but instead of utilizing just its internal components, Zn air cell breathing air to generate electrical power. To fully understand how this is achieve, check out this link to get the full detail.
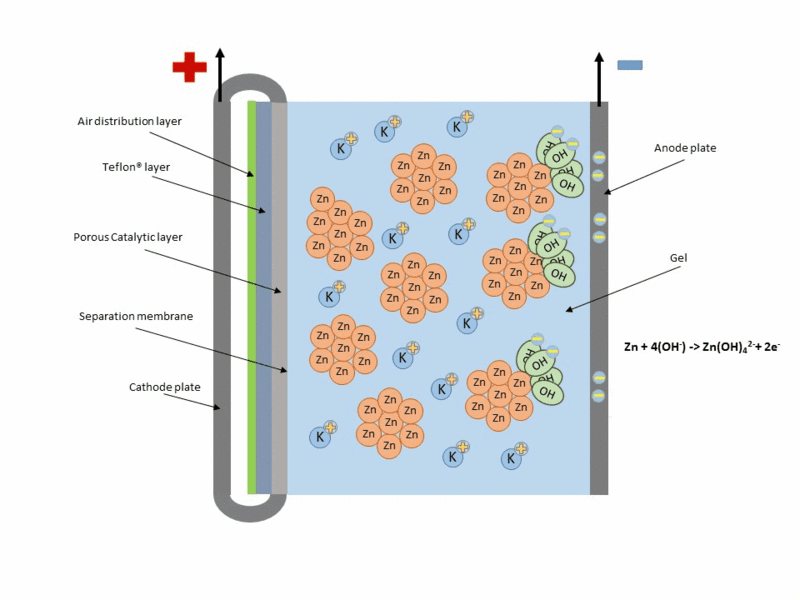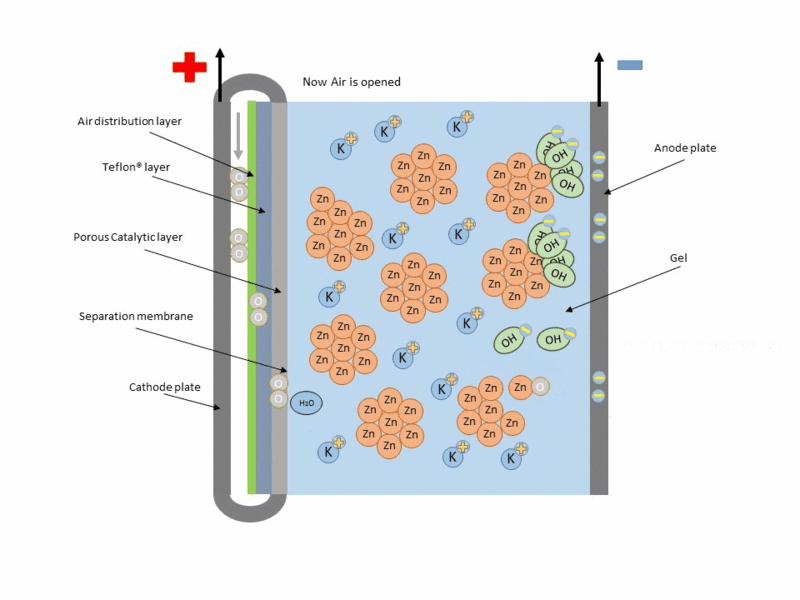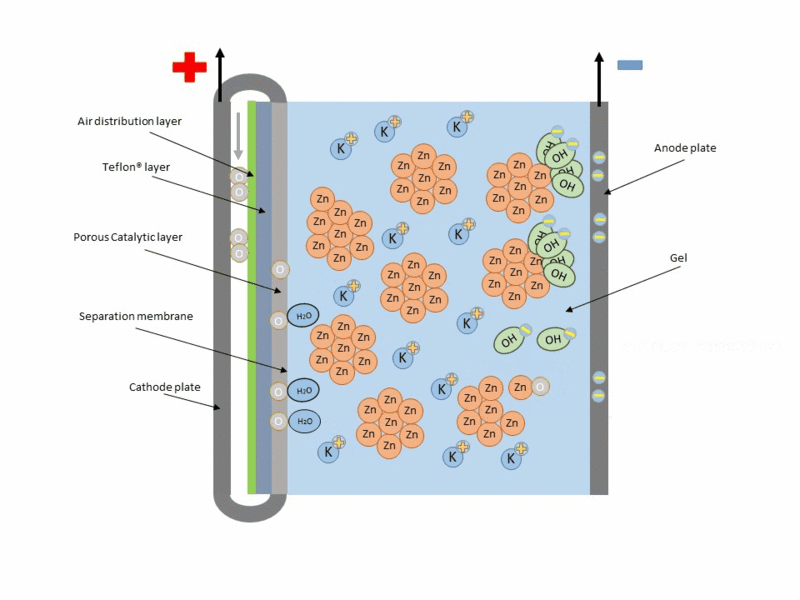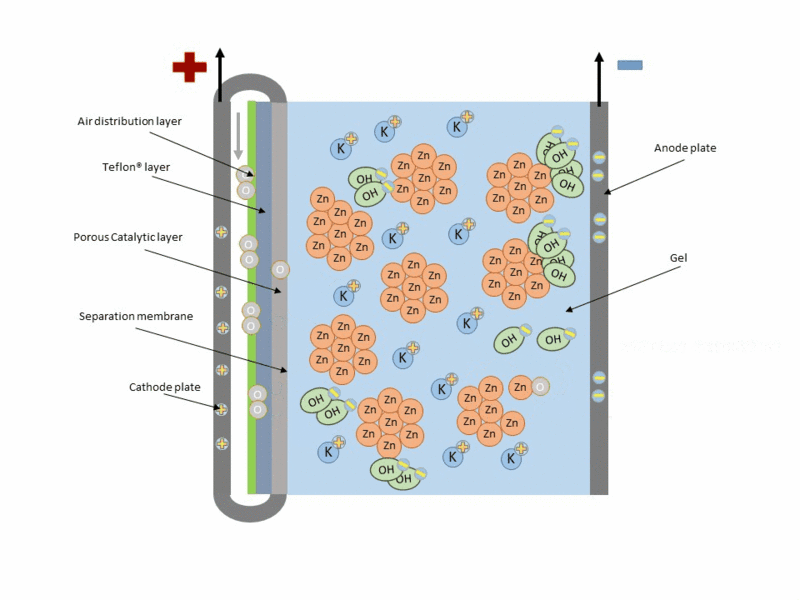
Working operation of zinc air cell Gif source: Wikimedia under CC BY-SA 3.0
Mind you, Zn air battery cannot be used in a air tight holder like other batteries since some air must coming first before energy can be produced.
But how then was the problem of rechargeability overcomed and what are the proves? I believed these are some of the questions running through your minds now, right? But worry not and be glad for the arrival of technological advancement and scientific research that has lead to the discovery of a new methodology for creating a bifunctional oxygen electrocatalyst that is currently used for manufacturing rechargeable zinc air battery from scratch; a discovery made and described by chemical engineering researchers from the University of Sydney (Australia) and Nanyang Technological University (Singapore).
A living proof of such breakthrough is the "holy grail"; a Air breathing zinc rechargeable cell produced by Nantenergy. When compared with Li-ion batteries, the company's zinc batteries offers longer duration at a lower cost, with absolutely no risks of overheating and fire. The interesting thing about this zinc air technology from Nantenergy is that its has been around for while now; having being deployed and currently in use in over a 1000 cell phone towers and 100 villages across Asia and Africa; providing a sustainable and reliable electrical power, especially to communities with little or no access to the grid. Aside the inferior lead-acid technology, zinc air cell has been able to overcome the 100$ per kWh barrier; a target that is yet to be accomplished by Li-ion technology.
Zinc air versus Lithium ion battery
Lithium ion technology is greatly responsible for virtually all environmental friendly technologies ranging from renewable energies, smartphones, tablets, personal computers, electric cars and many more but the problem with this battery is that when manufactured, it requires two rarely available metals; cobalt and lithium. The effect of cobalt mining on its surrounding environment is a sadden one especially in the Congo area. And further more, lithium metal can be very expensive with high tendency of overheating and when combined, it requires constant cooling in other to avoid fire outburst or explosion.
Zinc metal, on the other hand, is not only safe but is also among the most abundance minerals on the earth that is essential to the growth and proper functioning of the entire human body. The abundant nature of this metal has made the battery production much more cheaper than Li-ion battery and further more, they have higher energy storage capacity and are much more safer and more ecosystem friendly. Although, the chief material used (zinc), is a non toxic and can safely be disposed off, its mining process could present some potential hazards when carried out on a large scale.
With the current awareness and global availability of its materials, i believe this technology, if its production rate is to be scaled up to the likes of lead-acid or Li-ion battery, will in no time soon be commercially viable and widely recognized. I hope I have being able to justify my claim and you are able to learn one or two things from this write up.
Thanks for your time
REFERENCES
• Benefits and dangers of lithium ion
• Why rechargeable zinc air battery could become norm
• Power electronics; rechargeable zinc air could threaten the dominance of lithium battery
• Wikipedia; Electric battery


This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @utopian-io and @curie.
If you appreciate the work we are doing then consider voting all three projects for witness by selecting stem.witness, utopian-io and curie!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
An exciting piece of technology but the trait of being unable to be used in airtight conditions may limit the use of the battery in specific applications. But that may not be much of an issue in most RE application.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I really liked the topic of batteries since I got interested into smartphones and "techy stuff". Li-ion batteries are showing their age, and you can see that as new smartphone manufacturers give you big 4000+ mAh in each iteration. Seing new technologies for battery manufacturing is great and I hope it can be viable in the (near) future.
Nice one!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I hope so myself, especially at a reduced and affordable price
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
This is a wonderful write-up, a good way to transfer knowledge. It is my first time getting to know Zinc air battery and Nantenergy
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit