What? 20 years to develop a drug? You said There has not been single drug discovered in Ghana and that drug discovery is so expensive, that I could take, but 20 years of continuous work to come out with a single drug?
Well, that was before I was introduced to the world of pharmacy and drug discovery!
Today I want to introduce you to a company that is building a product which has caught my attention because of what it means to the world of medicine.
Meet FarmaTrust but don't be deceived by the farm in the name. This is one of the biggest ICOs and it is poised to transform the pharmaceutical industry. I will be talking more about this great innovation but before I do, let me take you through what goes on before you have a drug in your palm with a glass of water in hand.

source
The entire process that leads to the final product is engulfed in a blanket term known as drug development. Drug development usually involves three stages which are;
- Drug discovery / product development,
- Pre-clinical research and
- Clinical trials.
Drug discovery
Drug discovery is the process by which new medication candidates are found.
In the early days this often came about by accident and one can remember the discovery of penicillin by Alexander Fleming through contamination of plate culture.
Around the 1970s, drug discovery became more streamlined and involved targeting specific molecules which have been identified and demonstrated to play critical role in a particular disease.
Advancing medical studies and technology led to the understanding of the chemical bases of diseases which led more targeted drug discovery.
Deliberate modification or natural or synthetic molecules or genetic engineering leads to production of several thousands (5,000 to 10,000) of drug candidates which are then screened against a model of the disease/target or the infective microorganisms.
Drug molecules being sought for are those that can provide some therapeutic benefit after interacting with the target molecule.
The screening leads to the emergence of New Chemical Entities (NCEs) or New Molecular Entities (NMEs). Lead compounds which are the most promising substances are selected and this leads to the next stage!
At this stage, little will be known about the safety, toxicity as well as the mechanism of action of these compounds in humans.
Pre-clinical research
This is the stage after the drug discovery and before testing of drugs in humans can start.
Usually at this stage, living organisms such as microorganisms and animals are used. Animals mainly used include Rats, mice and dogs but monkeys and pigs may also be used.

source
The main goals of pre-clinical studies are;
- Determination of a product's ultimate safety profile.
- Preliminary study of the mechanism of action of the active compound.
- The pharmacokinetics in the test animals.
- Determination of the efficacy of the formulated drug product.
- Data compared with early clinical trials to learn how other animals and humans respond to the drug.
These results also determine toxicities to be monitored and the doses to be used in the clinical trials.
Clinical trials
At this stage human subjects are used and it involves four stages or better still four phases.
Phase I : This involves 20-80 healthy volunteers who would be used in trials. The main objective here is to check the drug's pharmacokinetics, pharmacology and safety.
Phase II: At the phase of the clinical trials, 100-200 patients are recruited. The main objective here is to get information on the drug's efficacy, adverse reaction and correct effective dose.
Phase III: This involves 100 to 1000s patients and the main objective is to get further information on efficacy, safety and overall risk-benefit of the molecule.
Phase IV: This is the last phase which involves a broader patient pop. Drug is usually approved by regulatory body and formulation then comes in.
Drug development can take average of 10 – 12 yrs and cost $ 900 – 2,000 million with some taken as long at 50 years a billions of dollars.
Now you may be wondering, why did I have to go through all the above with you?
Many people know about drug counterfeiting but not many know about the extent of damage this is causing the the entire chain of drug development from the developing company to the end user.
The steps above are all in place to protect the end user and this is enforced by regulations. So when the company spend this much money and time into developing the drug they are allowed usually a number of years of exclusive rights to the product. At this point, no other company can produce the same product without their permission. This is only fair because it allows them to recover their investment before other companies can join in.
Unfortunately not everyone wants to play fair. Other entities engage in illegal production usually of low quality which enable them to sell at lower prices. This results in huge financial lost to the company the company that discovered the drug and also pose medical threat to the end user.
In addition to the most tragic consequences of all, the problem bears witness of flaws in the pharmaceutical supply system and thereby undermines the credibility of the health and pharma industry. Furthermore, it represents huge lost profits. A report from 2014 states that counterfeit medicines “cost US businesses more than $200 billion annually and account for the loss of more than 750,000 jobs”.source WHO estimates the yearly cost at £36.9bn a year source.
StiboSystems Blog
It is estimated that more than 120,000 people die from counterfeit drugs yearly in Africa alone but the global figures are alarming.
Due to the devastating effects of drug counterfeiting, many agencies have been established to fight this as hard as possible. Unfortunately over the years, the fight even though having had results in some parts of the world has not generally been very effective.
How do FarmaTrust come in as a solution?
In order to solve the problem of drug counterfeiting the entire supply chain need to be sieved off its inefficiencies. This cannot happen with a centralized system and this is the reason why the fight against drug counterfeiting has not seen much results.
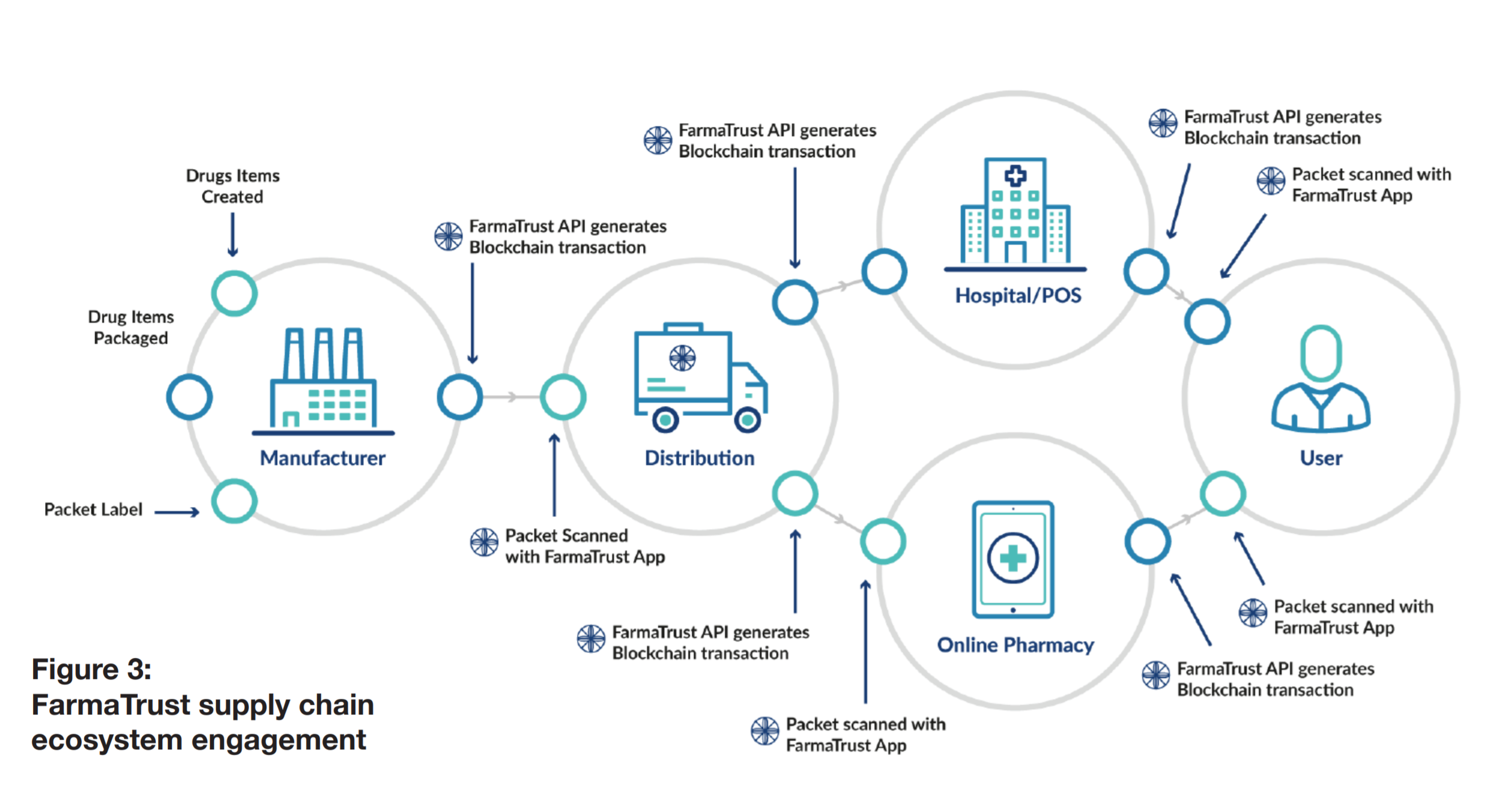
FarmaTrust is the most efficient global tracking system which provides security to the pharmaceutical companies, governments, regulators and the public, that counterfeit drugs do not enter the supply chain. Our Blockchain based system utilises Artificial Intelligence and big data analysis to provide the pharmaceutical industry with value added services which allow for more efficient processes and methods as well as a more transparent supply chain. Our system is safe, secure, encrypted and immutable.
FarmaTrust
The power of blockchain technology is been utilized to streamline the supply chain and weed out the inefficiencies thereby reducing supply chain cost as well as automating their reporting process. This will aid pharmaceutical companies to produce at low cost thereby leading to reduction in drug cost on the end user. The system will also eliminate the weak points utilized by counterfeiters as any end user can verify the product through the FarmaTrust application which is protected with the security of blockchain technology and completely decentralized.
You can check out this introductory video for more information!
FTT & ZOI Tokens
There are two different types of tokens on FarmaTrust platform which are the FTT and ZOI tokens. The ZOI token are for internal usage whereas the FTT tokens are exchangeable. However, the FTT tokens can be staked for the ZOI tokens on the FarmaTrust platform.
The Road map
starting in 2016, the team have gone through 9 major timelines and are currently rolling out the the last item on the 9th timeline which is: Start Agile Smart Contract Design & Development.
You can check out the front end demo of the FarmaTrust application.
Check out for project's whitepaper on their Official website.
This is my entry into a contest sponsored by FarmaTrust which is hosted by @originalworks and you can check it out HERE
farma2018
Hi,
Another great post. Easy to read. Let me suggest something that may help your readers a lot.
At the beginning of the post use
The difference will be huge. Just like on picture below:
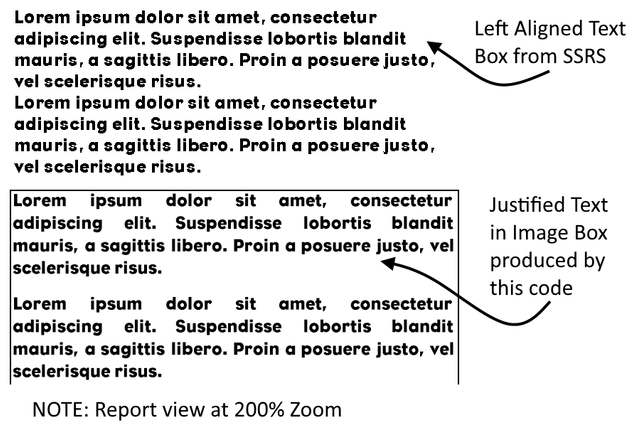
Good luck mate :) Obviously upvoted
Yours, Piotr
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
oh jezzz.
I just realized my silly mistake. In my previous post I didnt include the code that would justify text. Silly me.
Here is short article I've found that explain how to do it:
https://steemit.com/html/@dyrits/steem-it-1-how-to-justify-your-text
Oh and I also wanted to thank you for your comment in my latest contest. I appreciate your time and support. Cheers! :)
Yours,
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Wow ! as if you knew, I wanted to inquire about how you did it with your article and bam you have shared it....Thanks mate and I am checking it out right away!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
i really hope it worked :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Submitted.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit