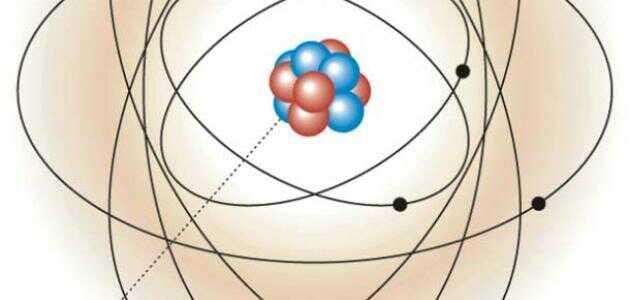
charge is neutral, or it can be said that it has no charge at all. Neutrons are present in the nucleus of the atom, which is a high-density region in the center of the atom. It is full of neutral-charged protons and positively charged neutrons, The neutron charge is connected to protons in the nucleus of the atom by a powerful force called nuclear power. Although the neutron does not affect the total atom charge, it has many other properties that affect the atom. Neutron Neutron is an atomic particle found in the nuclei of all atoms except the ordinary hydrogen atom. The neutrons are called the nucleon with the protons in the nucleus of the atom, and the neutrons are equal to 1.67493 x 10-27 kg, Which is about 1839 times larger than the mass of the electron, but is about 0.2% larger than the mass of the proton. Together, the proton and the neutrons form 99.99% of the mass of the atom. The mass of the neutron is equal to the sum of the electron and proton masses. Protons with neutrons, can Use the atomic mass equation and the atomic number to find the number of neutrons in the atom.
Authors get paid when people like you upvote their post.
If you enjoyed what you read here, create your account today and start earning FREE STEEM!
If you enjoyed what you read here, create your account today and start earning FREE STEEM!