It is not uncommon for people to seek to extract salts from seawater, since different salts and minerals abound in it, the most abundant salt is in fact sodium chloride or common salt, but although this is very easy to obtain, since it is enough to subject seawater to evaporation to obtain a brine, extracting other salts requires more complex processes due to their low concentration or high solubility, but many researchers are looking for simple ways to isolate these salts, since they can constitute an economic raw material for valuable metallic elements, such as lithium, magnesium and potassium, among others.

New method would use seawater as raw material to obtain magnesium salts. Source: edited image, original from pxhere.com.
Nowadays, isolating magnesium salts from seawater has aroused great interest, since they are a raw material for obtaining magnesium metal, a metal with many emerging applications, such as new-generation batteries and carbon capture.
Magnesium is part of the alkaline earth metals, and has been known since ancient times for being a light metal and for its great capacity to form resistant alloys, and from the chemical point of view its behavior is very similar to that of sodium, its neighbor in the periodic table, so it has high reactivity and ease of forming positive ions, making it very attractive for different applications in the chemical and metallurgical industry. That is why magnesium is counted as a strategic material in many nations.

Crystalised magnesium. Source: Wkipedia.org.
But, although magnesium is very abundant in seawater, being the third most abundant dissolved element after sodium, it is not so easy to extract magnesium from seawater, it is not so affected by temperature and its salts have a high solubility in water. The extraction of magnesium from seawater involves the addition of calcium oxide as a precipitating agent, magnesium sulfates and chlorides react with calcium oxide to produce magnesium hydroxide, the slurry formed is allowed to settle to the bottom, and then the solids are separated, filtered and washed to remove the chlorides.
The precipitation reaction can be written as:
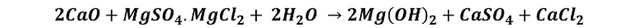
The resulting cake containing the magnesium hydroxide obtained as a product is then calcined in furnaces at 1100 °C to obtain MgO. However, the coexistence with calcium cations (Ca2+) inevitably leads to the formation of impurities such as CaCO3, so other more complex purification processes are then required. Another way to obtain magnesium is by electrolysis of magnesium chloride.
Because of these complex and energy-intensive processes, magnesium extraction research is moving towards finding simpler and more sustainable methods, and researchers at the Pacific Northwest National Laboratory (PNNL) and the University of Washington (UW) appear to have found a simple way to extract a pure magnesium salt, which could serve as a feedstock for magnesium metal.
According to the article recently published in the journal Environmental Science & Technology Letters, the new method consists of flowing two solutions, side by side, through a long laminar flow stream, taking advantage of the fact that the fresh solutions flow without allowing the system to reach equilibrium, creating a constant reaction interface at the boundary separating the two solutions. This procedure was called the laminar co-flow method.
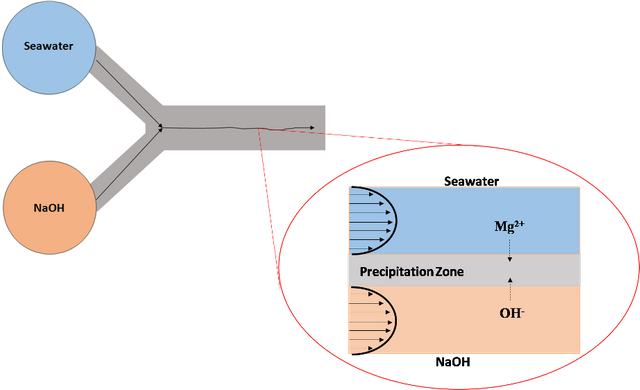
Schematic showing reactive ions at the laminar co-flow interface. Source: Image prepared in Powerpoint.
By this method, the researchers were able to determine that non-equilibrium conditions in laminar co-flow, using a microfluidic device designed for the experiment, in which a NaOH solution and a seawater solution are injected, resulted in a much higher selectivity for obtaining Mg(OH)2, compared to the conventional method described above, since by flowing the seawater together with the sodium hydroxide solution, the magnesium-containing seawater reacts rapidly forming a layer of solid magnesium hydroxide, which then acts as a barrier preventing the mixing of the solution.
The solids obtained were characterized for their composition, allowing both the purity of the precipitates and the yield of the process to be optimized solely by systematic variations of the NaOH solution concentration and reaction time.
Although it was previously known that magnesium hydroxide salt could be obtained from seawater by mixing it with sodium hydroxide, which can then be processed to obtain magnesium metal, the problem is that this reaction pathway gives a complex mixture of magnesium and calcium salts that are difficult to separate.
In this sense, this method provides a simpler way to obtain a magnesium hydroxide solution in a more sustainable and pure way than the previously used methods, advancing research towards more energy efficient ways with better yields, instead of achieving more complex processes that consume more resources.
Thanks for coming by to read friends, I hope you liked the information. See you next time.
References
Wikipedia.org. Magnesium.
UANL Facultad de Ingeniería Mecánica. Métodos de obtención de óxido de magnesio
Qingpu Wang, Elias Nakouzi, Elisabeth A. Ryan, and Chinmayee V. Subban (2022). Flow-Assisted Selective Mineral Extraction from Seawater. Environmental Science & Technology Letters, 9 (7), 645-649.

@tipu curate
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit