As we well know there is a race that does not stop in the development of new batteries, while it is true that lithium-ion batteries made possible greater autonomy in electric devices and mobility on board electric vehicles, however a more efficient and sustainable technology that can replace it is incessantly sought. But could sodium be the main element to replace lithium in batteries?

Source: image created in powerpoint, contains an image from pixabay.com.
Sodium as an element is much more abundant than lithium, it can be extracted from the ocean and the earth's crust, which makes it economical and sustainable, very desirable characteristics for battery components, however sodium batteries have not been able to supply as much energy as lithium batteries, and have stability problems, so this technology has not been considered as a strong candidate to replace the dominant lithium batteries, but this could change.
Despite the stability and energy density of lithium batteries, their main disadvantage is that their main components, lithium and cobalt, are scarce and expensive. And as the demand for electric vehicles increases, manufacturers of batteries for these vehicles will need cheaper and more accessible materials to meet the demand, as lithium and cobalt will become more difficult to obtain. And since lithium and sodium are in the same group on the periodic table, as part of the alkali metals, this suggests that they may have similar characteristics that can be used to make batteries.
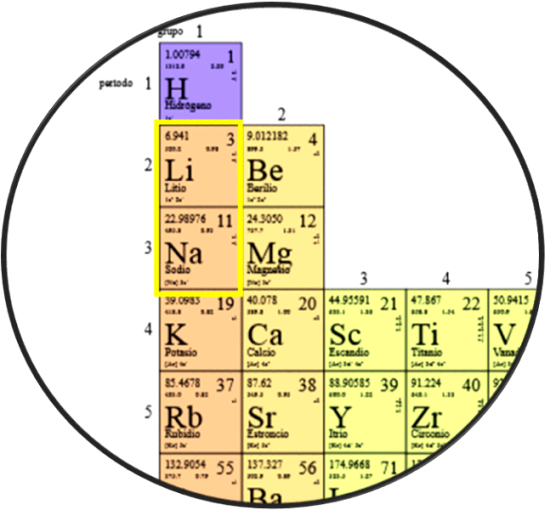
Sodium and lithium would have common properties for use in batteries. Source: edited image, original taken from Wikimedia.org.
What is a sodium battery?
We could say that a sodium battery is a rechargeable battery that works in a similar way to a lithium battery, with the difference that the charge transport is carried out by sodium ions (Na+) instead of lithium ions (Li+).
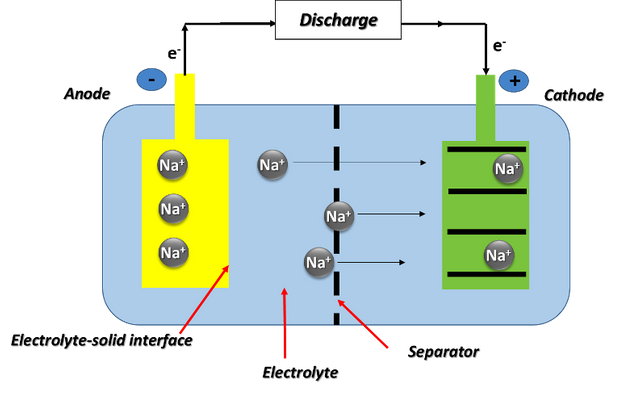
Diagram of the operation of a sodium-ion battery. Source: image elaborated in powerpoint.
Basically, sodium ion cells consist of a cathode made of a sodium-containing material, an anode that does not necessarily contain sodium, and a liquid electrolyte containing sodium salts dissociated in polar solvents. During the charging cycle, sodium ions are removed from the cathode and inserted into the anode, while electrons travel through the external circuit; and during discharge, sodium ions are removed from the anode and reinserted into the cathode, while electrons travel through the external circuit.
In other words, the battery components and the electrical charge storage mechanism are basically the same, except for the ionic carriers in each.
In fact, sodium battery technology is not new, even its study began between the 70s and 80s, in parallel to the development of lithium batteries, but due to the success and rapid progress of commercial applications that used lithium technology, this system was eventually abandoned, that coupled with the technology of the time did not allow the observation and proper handling of sodium, limiting the performance of these batteries.
However, in the 80's, before lithium-ion batteries started to be commercialized, some American and Japanese companies developed sodium batteries as complete cells that used a sodium-lead alloy as anode and a NaxCoO2 compound as cathode, which despite having a remarkable cyclability, delivered an average voltage of less than 3 V, which did not attract attention compared to the 3.7 V delivered by LiCoO2 batteries.
On the other hand, Na+ ions (1.02 Å) are larger than Li+ ions (0.76 Å), which ultimately affects the transport properties and the formation of the solid-electrolyte interface. Sodium is also heavier than lithium and has a higher standard electrode potential (-2.71 V versus -3.02 V for lithium); so the sodium-based system falls short in terms of energy density. But, driven by the growing demand for rechargeable batteries, the last decade has seen a revival of interest in sodium batteries.
In search of the right electrolyte
Recently a research team at Pacific Northwest National Laboratory has developed a sodium-ion battery with far superior stability in laboratory tests. An ingenious change in the electrolyte components avoids the solvation problems at the solid-electrolyte interface that limit the performance of sodium batteries.
In any battery, the electrolyte is the medium that maintains energy flow; it is formed by dissolving salts in a suitable solvent, giving rise to charged chemical species that flow between the electrodes, establishing energy flow. But, with repeated charge and discharge cycles, the electrochemical reactions taking place in the electrolyte slow down or appear as side reactions that eventually decrease the performance of the batteries to the point where they can no longer be recharged, and this effect appears faster in sodium batteries than in lithium batteries.
But this research team designed an electrolyte capable of suppressing the dissolution of the solid-electrolyte interface, reducing the solvation capacity of the electrolyte and improving the performance of the sodium battery. The complete cell developed consists of a hard carbon (HC) anode and a NaNi0.68Mn0.22Co0.1O2 (NaNMC) cathode, abbreviated as HC||NaNMC, and with the new electrolyte they demonstrated that the cell maintained 90% of its capacity after 300 cycles when charged at 4.2 V; a very high value compared to previous records for most sodium batteries.
And although sodium battery technology still lags behind lithium-ion batteries, thanks to its greater advantages and the development of new electrolytes it could in the future become the most suitable option for light electric vehicles and energy storage from renewable sources.
Thanks for coming by to read friends, I hope you liked the information. See you next time.

In Syria, we use Lithium batteries for electricity cuts but they are very expensive which makes them unreachable for most people...This is the first time I hear about Sodium batteries, and from my reading, I think it is going to be a huge deal for the entire battery industry...Thanks for sharing :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Hello friend, that's the idea, to lower the cost of batteries with more economical and sustainable elements, because lithium batteries will only continue to become more and more expensive.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Hi @emiliomoron
If a battery is finally achieved that works with Sodium, and is stable and long lasting, costs would decrease, even the pollution levels produced by batteries that are waste at the end of their useful life.
I hope this will finally materialize to be a big step.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Hi @josevas217, it would certainly be a great step and would certainly contribute not only to reduce costs but also to produce less hazardous waste.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Hi friend, it would turn out to be very interesting if this sodium battery proposal would reach all the expected standards.
Greetings and thanks for this valuable post.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
It is certainly a technology with many advantages, it would certainly be very interesting to see it in operation, let's hope that its development continues and we can see its implementation.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit