 Do you ever wonder,"can my smartphone ever stay a minute longer" well it can,if you know the mysteries of Batteries.
Do you ever wonder,"can my smartphone ever stay a minute longer" well it can,if you know the mysteries of Batteries.
Batteries allows us to use a lot of gadgets and appliances such as smartphones and power banks without anchoring us to an entangling of power cables,Even the best batteries would diminish slowly,losing capacity until they finally run down.
You must be wondering, How are batteries storing so much electric charge? Well it all started with a little history on batteries.

It all started with two italian scientists,
.jpeg)
luigi Galvani and
.jpeg)
Alessandro Volta and even a frog in 1780,they both argued about what happened to the frog.
It's is said that as Galvani was studying a frog's leg,he brushed a metal instrument against one of it's nerves, making the leg jerk,Galvani then called this Animal Electricity cause he believed that a type of electricity was stored in the very life of the frog, but Volta disagreed stating that it was the metal itself that made the metal itself that made the leg twitch.
The argument was eventually resolved with Volta experiment (known today).
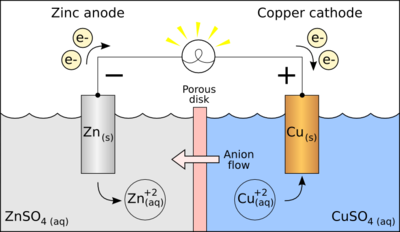
Alessandro Volta tested is idea with a stack of alternating layers of zinc and copper which were separated by a piece of paper or clothing which has been soaked in a salt water solution(we call this the Volta's cell or voltaic pile). What happened in the Volta's cell is known as :
- Oxidation
- Reduction
The zinc oxidises which means it loose electrons which are transferred and gained by ions in the water,the process called reduction.
The oxidation and reduction cycle creates flow of electron between two substances and if you put an electrical appliance like a light bulb between them,the appliance is been powered up.
 source
sourceSince then, scientists have improved on Volta’s design. They've replaced the chemical solution of salt solution with dry cells filled with chemical paste, but the principle remains the same.
Principle being that a metal oxidises and sends electrons to do some work before they are regained by a substance being reduced. But any normal battery has a limited supply of metals being oxidized,and when most of it has been oxidized,the battery dies,so this brings us to rechargeable batteries.


Rechargeable batteries has a temporary solution to this problem that any normal batteryhas by making the Oxidation-Reduction process reversible.
The reversibility makes it so that electrons would flow back in the opposite direction with the application of Electricity, Plugging in a charger to the battery and the outlet which draws the electricity from the wall outlet and drives the reaction to regenerate the metal,making more electrons available for oxidation the next time to be used.
Main problem is that,even rechargeable batteries don't last forever,cause overtime,the repetition of this process prevents the battery from oxidizing properly,this causes irregularities on the metal surface,so therefore electrons are not available to flow through the metal surface (and so the battery dies permanently). Some everyday rechargeable batteries will die after just hundreds of discharge-recharge cycles,while newer advanced batteries such as smartphones and digital cameras *batteries” can survive thousands of discharge-recharge cycles.
But some cars charge while on motion or by using solar,and until this can be incorporated on simple normal batteries,we should all be hoping for a breakthrough in the future of batteries or continue to depend on electric charging for the meantime.
.jpeg)
@Gbindinazeez, thank you for using the naijapidgin tag.
We encourage and support minnows.
Join us on discord: https://discord.gg/5SR8CH4 for more fun and to submit your posts for curation.
You like what we are doing and would like to support us? Join our trail here: https://steemauto.com/dash.php?i=15&id=1&user=naijapidgin
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by gbindinazeez from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit