(Tábita Hünemeier et al. / Science Advances, 2023 https://bit.ly/42e6Czg)
Researchers from the University of São Paulo have found that the indigenous peoples of the Amazon rainforest are practically free from American trypanosomiasis, the Chagas disease.
Subsequent genomic analysis revealed several mutations in the tribes that protect them from infection with Trypanosoma cruzi, the causative agent of the disease.
According to the World Health Organization, Chagas disease, or American trypanosomiasis, affects six to seven million people worldwide.
Most people get sick from contact with triatomine bugs that carry the pathogen Trypanosoma cruzi.
South and North America are considered the main region of the disease, and it has begun to penetrate more and more cities.
Because of this, it has become more common in the US and Canada.
THE STUDY
There is no vaccine against this disease, so all measures are aimed at preventing transmission of the infection:
- fighting against bedbugs
- screening the blood of donors
- screening women of childbearing age.
However, as the researchers found out, some people have developed adaptive mechanisms that protect them from Chagas disease.
They examined the genome of 118 representatives of 19 tribes living in the Amazon region, in which the disease was practically not recorded.
Scientists have found that people from these tribes have mutations in the genes:
- PPP3CA
- DYNC1I1
- NOS1AP
The most common “defender” turned out to be the PPP3CA gene, which encodes one of the most important elements of intracellular G-protein signal transduction in immune cells.
In addition, the gene is responsible for calcineurin signaling, a key conduit for innate immunity against Trypanosoma cruzi.
In disease-resistant people, this gene turned out to be inactive.
To test the role of this gene in the development of Chagas disease, the researchers infected cardiomyocytes derived from human induced pluripotent stem cells infected with T. cruzi.
The team led by Tábita Hünemeier chose cardiomyocytes as the infection model because the parasite often infects these cells in humans.
They found that in cells where PPP3CA gene expression was reduced by 65% compared to control, the parasite's ability to infect cells was significantly reduced by about 25%.
THE IMMUNE
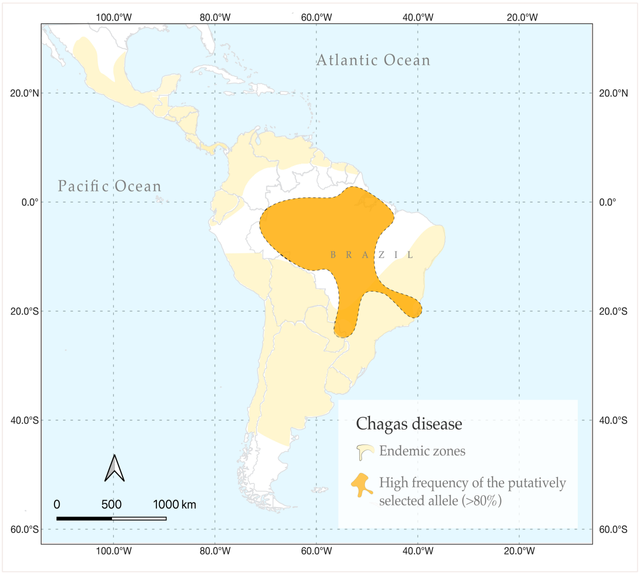
The team also studied the geographical distribution of both Chagas disease and the mutation in the PPP3CA gene.
They found that there are practically no cases of trypanosomiasis in the distribution area of the mutation, which also indirectly proves its protective effect.
Hünemeier’s team believes that this natural selection of the mutation occurred for several reasons.
First, the selection process began about seven thousand years ago, judging by the archaeological finds, which indicates a sufficient time for selection.
Secondly, the geographical features of the region under study make it possible to construct a model in which isolation led to the persistence of the mutation.
Third, the fact that this mutation occurs in similar environmental conditions in Africa and southern Europe suggests that in the studied region, environmental conditions are suitable for the mutation to persist.
Sources:
- Science Advances: https://www.science.org/doi/10.1126/sciadv.abo0234
- Technology Networks: https://www.technologynetworks.com/tn/news/amazonian-populations-have-genetic-protection-against-chagas-disease-370944
Wanna relax, sleep or improve your focus?
Check this rain sound video: https://bit.ly/rainsfocus
Thank you, friend!


I'm @steem.history, who is steem witness.
Thank you for witnessvoting for me.
please click it!
(Go to https://steemit.com/~witnesses and type fbslo at the bottom of the page)
The weight is reduced because of the lack of Voting Power. If you vote for me as a witness, you can get my little vote.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Upvoted! Thank you for supporting witness @jswit.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit